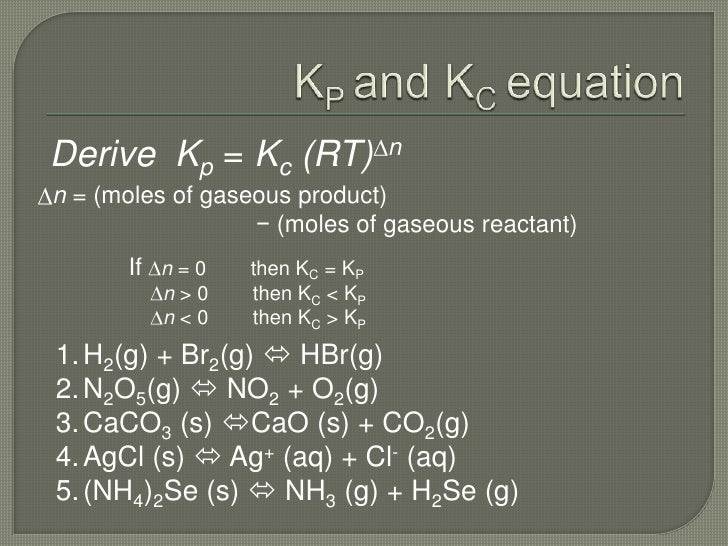
Kp Kc Rt N
So you have kp equals kc times rt to the delta n. Mol product tot.
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcriutd8t8gp6eegucf3hcmsyv4yyzrpgoj Ra6 Qqmdybvtieks Usqp Cau
N 2 o 4 l is an important component of rocket fuel at 25 circ c n 2 o 4 is a colorless gas that partially dissociates into no 2 the color of an equilibrium mixture of these 2 gasses depends on their relative proportions which are dependent on temperature.
Kp kc rt n. Above the symbols represent. The derivation of this expression uses the ideal gas equation so use b. Equilibrium is established in the reaction n 2o 4 g rightleftharpoons 2no 2 g at 25 c.
Converting between kc and kp using kp kc rt delta n. Now you re never going to be asked about the derivation of the kc kp relationship so this might be a situation like 2 where some simple rote learning of the pairings is best sufficient. Notice how it s pressure so this deals with pressures in atmospheres.
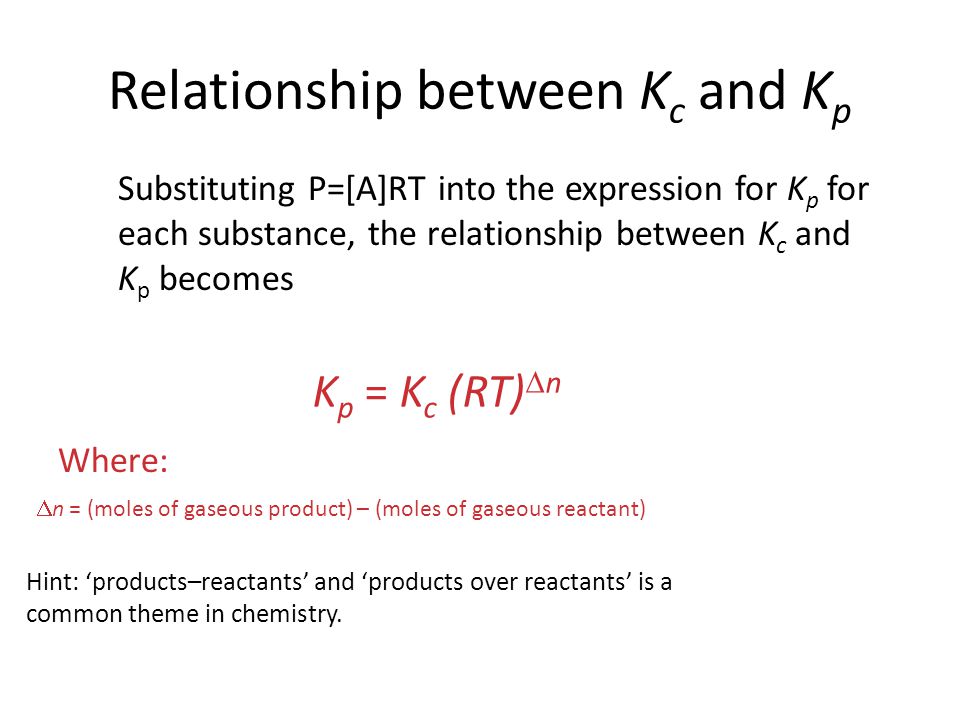
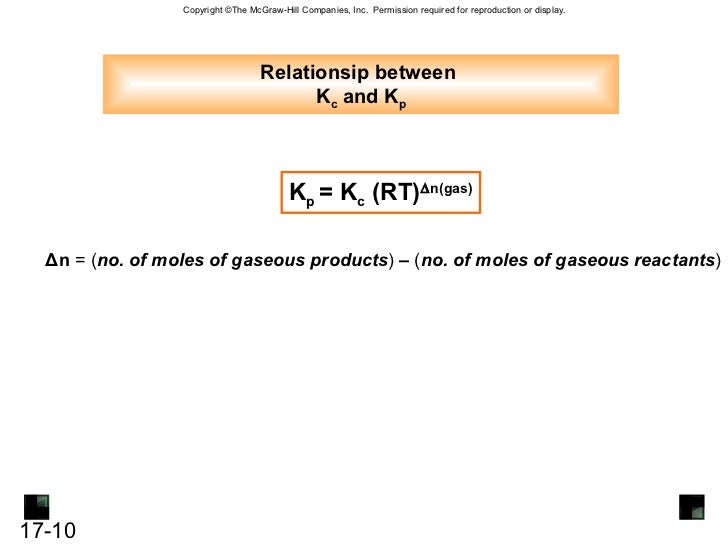
Where kp is the equilibrium constant of pressure kc is the equilibrium constant of concentration r is the universal gas constant 8 314 jmol 1 k 1 t is the temperature and δn is the difference between total moles of gas products and the total moles of gas reactants. Kp equilibrium constant using partial pressures. Kp kc rt d n.
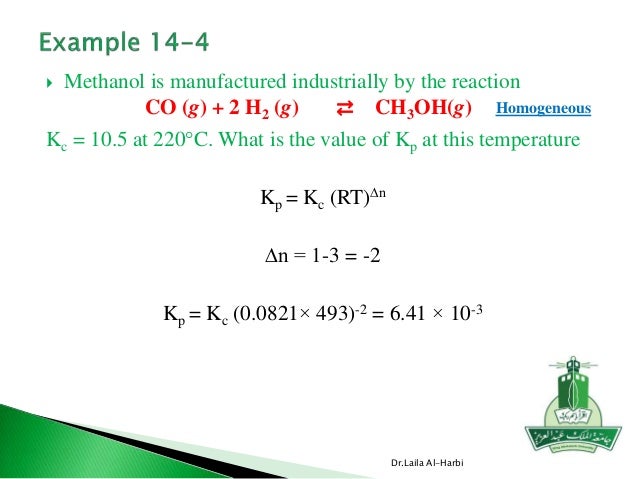
R 0 0821 when using atm as pressure units. For 2so 3 g 2so 2 g o 2 g kc 4 08 x 10 3 at 1000 k. δ n is the change in moles.
R 0 0821 l atm mol k. So using our example kp would have been equal to the pressure of no2 gas and that quantity squared over the pressure of n2o4 gas. Kc is the equilibrium constant for molar concentration.
D n tot. Kp is the equilibrium constant and pressures. What is the difference between kc and kp.
T is temperature in kelvin. Kp kc rt d n 4 08 x 10 3 0 0821 x 1000 1. Kp kc rt δ n.
This the exponent of r and t multiplied together. T temperature in k. D n number of moles of gaseous products number of moles of gaseous reactants.
Kp kc rt δ n. K p k c rt dn d n is the difference in the number of moles of gases on each side of the balanced equation for the reaction. Kp is the equilibrium constant for pressure.
R is our old friend the gas constant from the ideal gas law section.
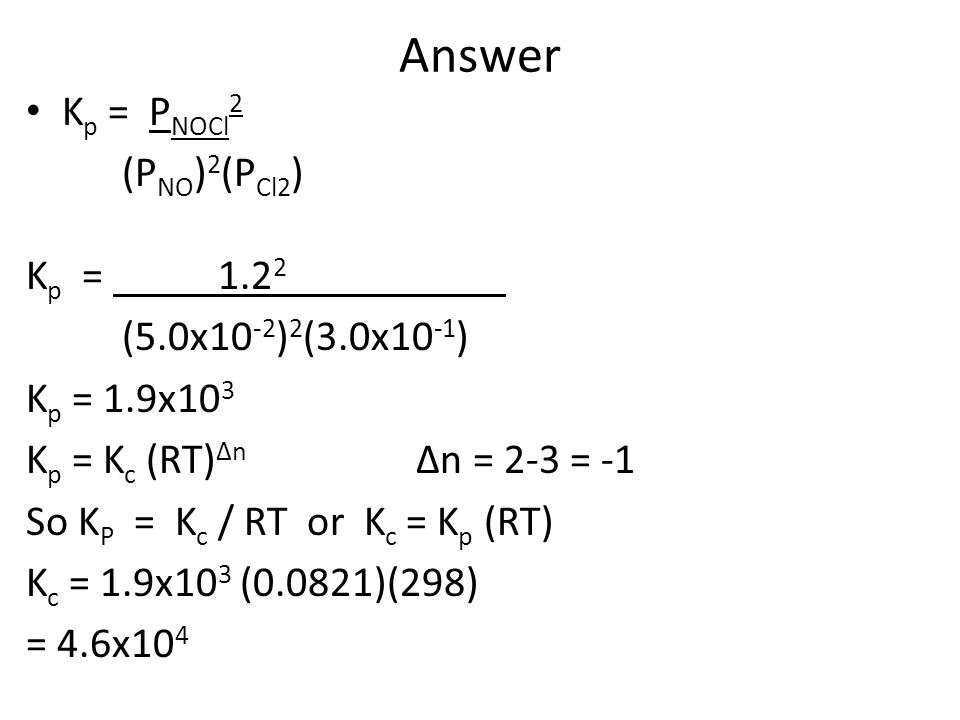 Equilibrium Follow Up Ppt Video Online Download
Equilibrium Follow Up Ppt Video Online Download
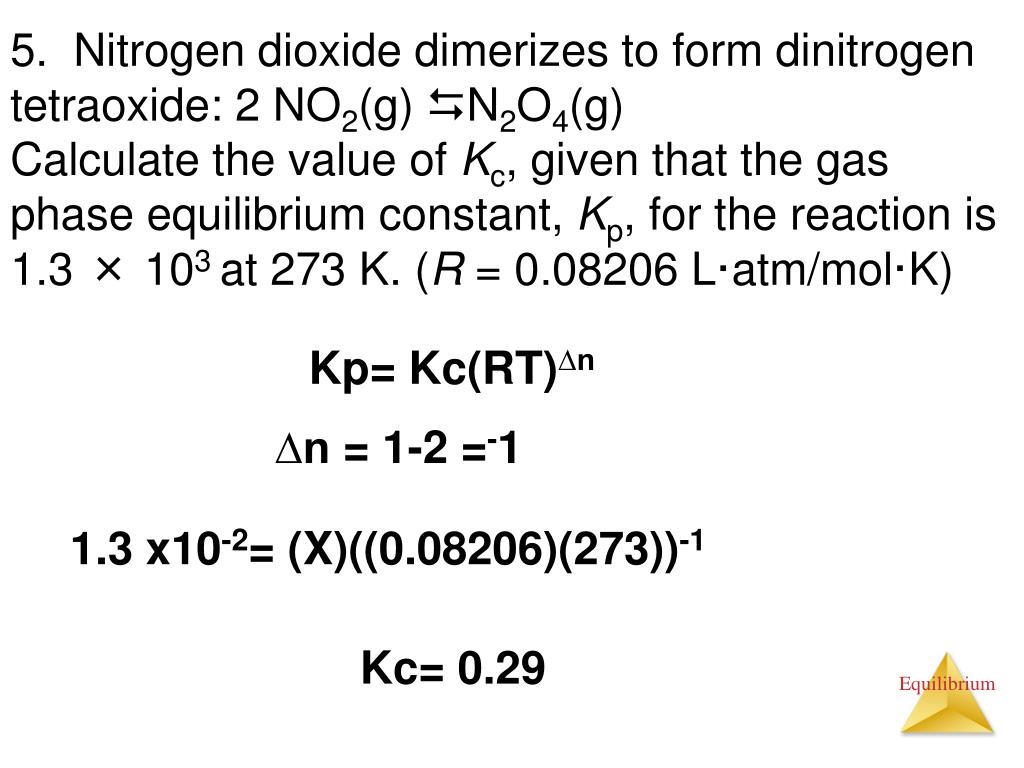 Ppt Entry Task Jan 8 Th Tuesday Powerpoint Presentation Free
Ppt Entry Task Jan 8 Th Tuesday Powerpoint Presentation Free
 Chapter 15 Overview Equilibrium Reactions Equilibrium Constants K
Chapter 15 Overview Equilibrium Reactions Equilibrium Constants K
 How To Calculate Kp Given Kc Equation Formula Youtube
How To Calculate Kp Given Kc Equation Formula Youtube
What Are Kp And Kc What Is Relation Between Them Quora
What Are Kp And Kc What Is Relation Between Them Quora
 Relation Between Kc And Kp Youtube
Relation Between Kc And Kp Youtube
 When Are Kp And Kc Values Equal Chemistry Stack Exchange
When Are Kp And Kc Values Equal Chemistry Stack Exchange
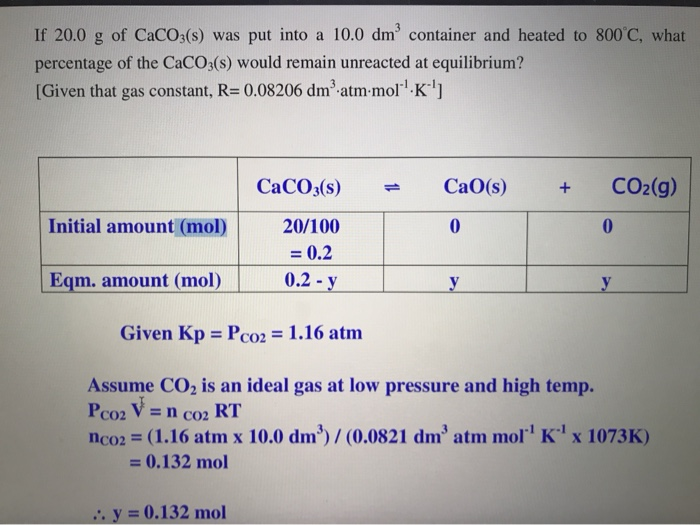
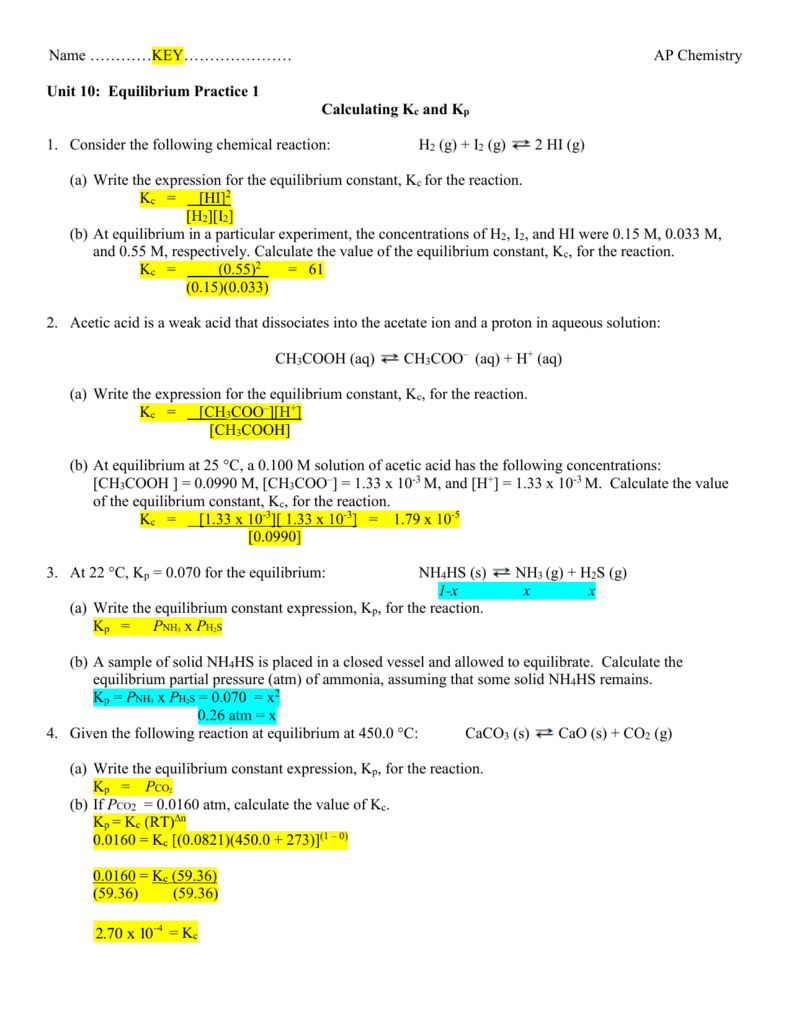
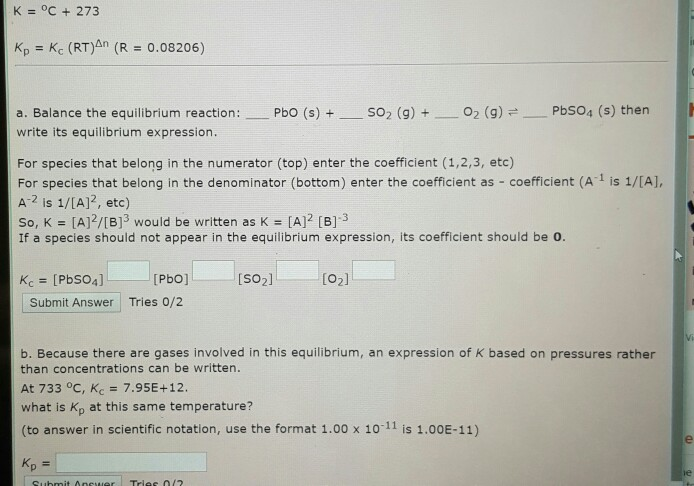 Solved K Oc 273 Kp Kc Rt N R 0 08206 A Balance T
Solved K Oc 273 Kp Kc Rt N R 0 08206 A Balance T
 Students Should Be Able To 1 Use The Equilibrium Constant
Students Should Be Able To 1 Use The Equilibrium Constant
 Chapter 14 Chemical Equilibrium Ppt Video Online Download
Chapter 14 Chemical Equilibrium Ppt Video Online Download
What Is The Relationship Between Kp And Kc Quora
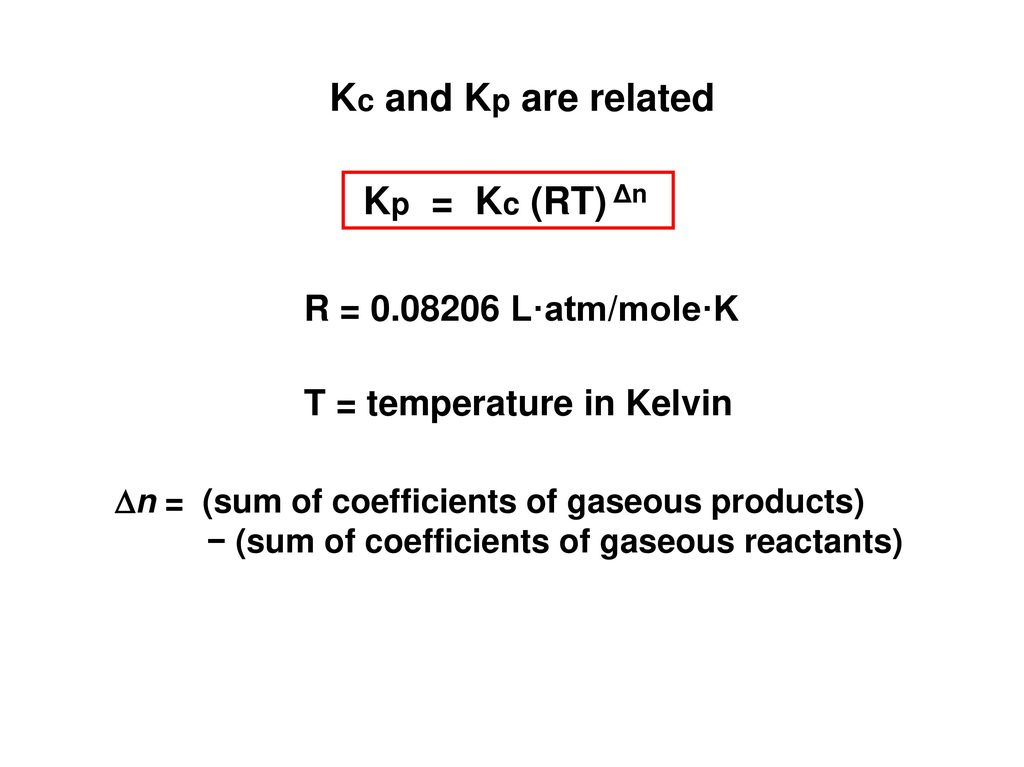 Chemical Equilibrium 2 Opposing Reactions Occur Simultaneously
Chemical Equilibrium 2 Opposing Reactions Occur Simultaneously
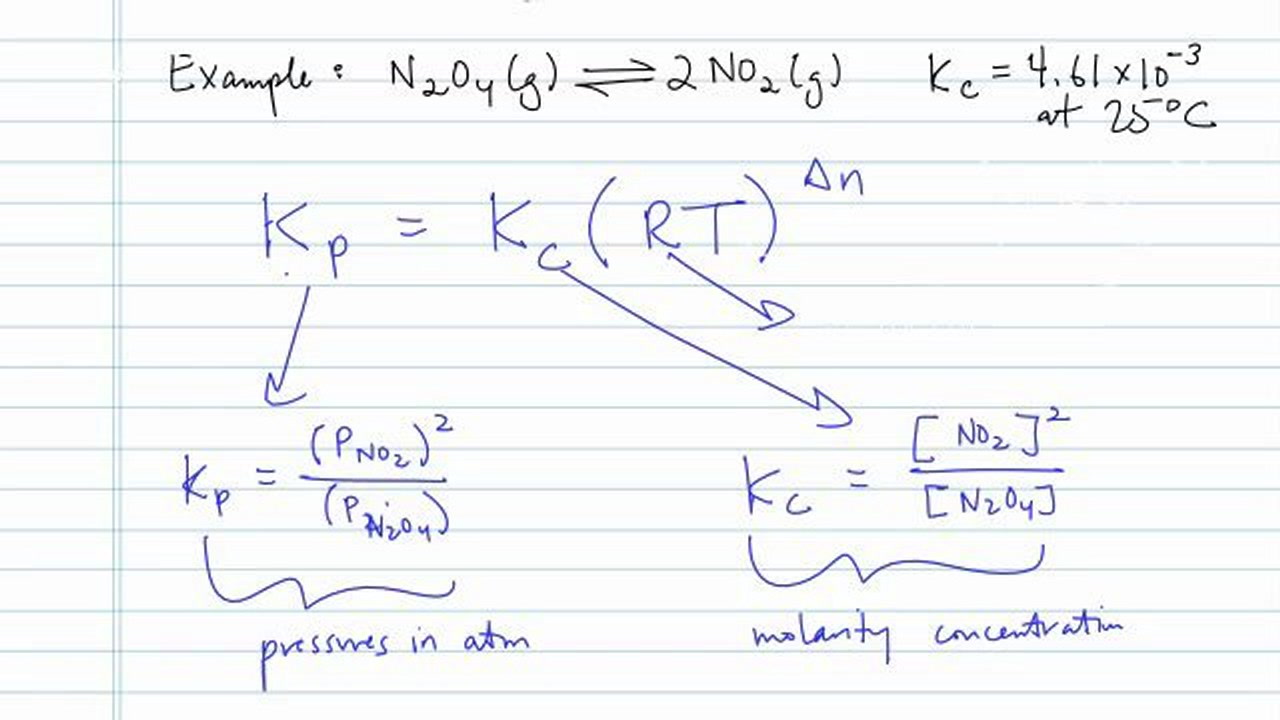 Tips For Converting To Kp From Kc Concept Chemistry Video By
Tips For Converting To Kp From Kc Concept Chemistry Video By
 Solved Why The Equ Kp Kc Rt Delta N Cannot Use In This Qu
Solved Why The Equ Kp Kc Rt Delta N Cannot Use In This Qu
 Kc And Kp Conversions Hess S Law In Equilibrium Constants Ppt
Kc And Kp Conversions Hess S Law In Equilibrium Constants Ppt
 Q 22 Kp Kc When A The Reaction Is At Equilibrium B The
Q 22 Kp Kc When A The Reaction Is At Equilibrium B The

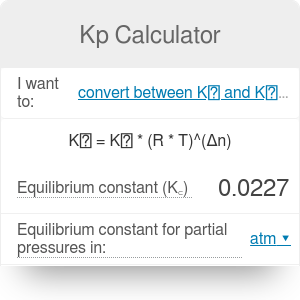 Kp Calculator Equilibrium Constant Omni
Kp Calculator Equilibrium Constant Omni
 Ppt Entry Task Jan 8 Th Tuesday Powerpoint Presentation Free
Ppt Entry Task Jan 8 Th Tuesday Powerpoint Presentation Free
 What Are Kp And Kc What Is Relation Between Them Quora
What Are Kp And Kc What Is Relation Between Them Quora
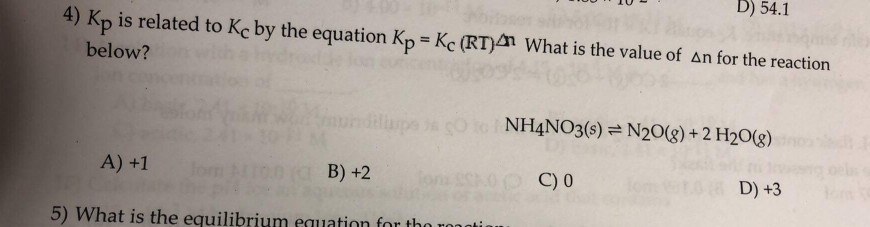 Solved D 54 1 4 Kp Is Related To Kc By The Equation Kp
Solved D 54 1 4 Kp Is Related To Kc By The Equation Kp
What Are Kp And Kc What Is Relation Between Them Quora
 Ppt Entry Task Jan 8 Th Tuesday Powerpoint Presentation Free
Ppt Entry Task Jan 8 Th Tuesday Powerpoint Presentation Free
 Solved Do I Need To Use Kp Kc Rt Delta N Confused Chegg Com
Solved Do I Need To Use Kp Kc Rt Delta N Confused Chegg Com
 Equilibrium Iv Kp Vs Kc Youtube
Equilibrium Iv Kp Vs Kc Youtube
 Chemical Equilibrium Chapter Ppt Video Online Download
Chemical Equilibrium Chapter Ppt Video Online Download
 Ap Lecture Derivation Of Kp Tp Kc Youtube
Ap Lecture Derivation Of Kp Tp Kc Youtube
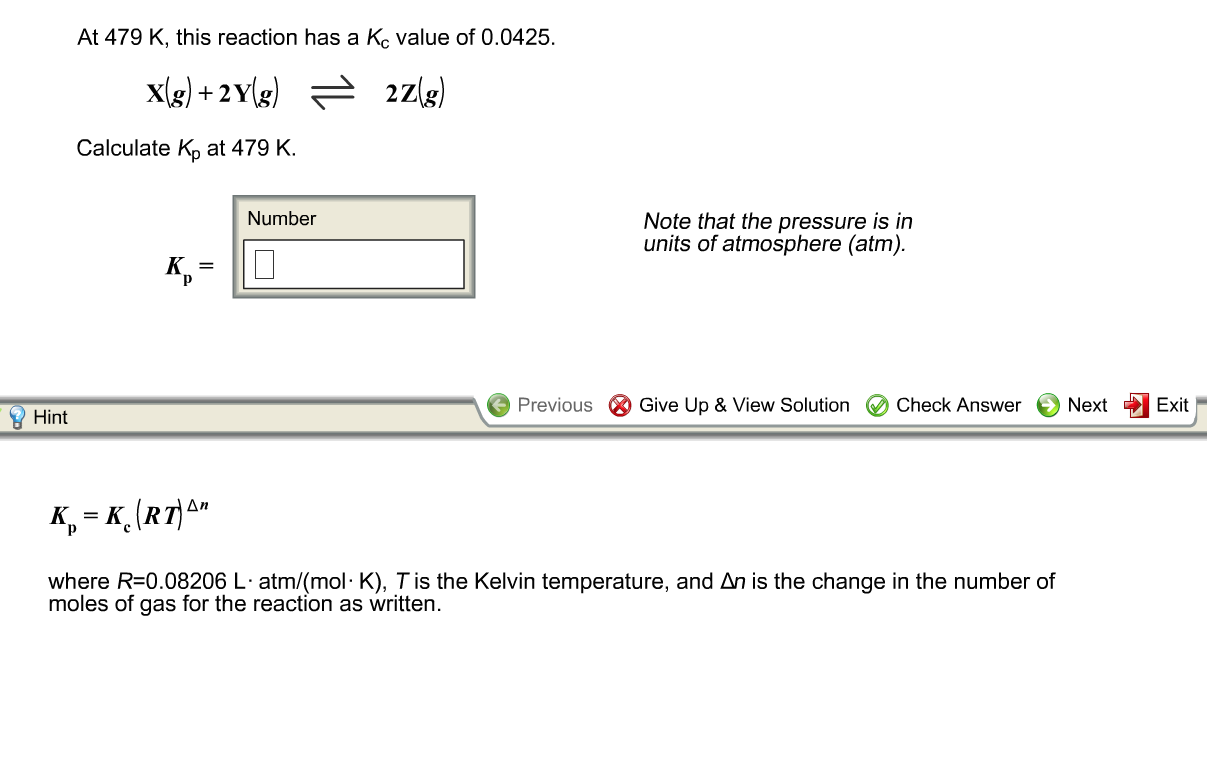 Solved At 479 K This Reaction Has A Kc Value Of 0 0425
Solved At 479 K This Reaction Has A Kc Value Of 0 0425
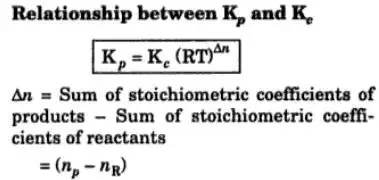 What Are Kp And Kc What Is Relation Between Them Quora
What Are Kp And Kc What Is Relation Between Them Quora
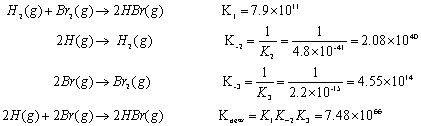

Posting Komentar
Posting Komentar