Ionization Energy Vs Electron Affinity
Note that ionization energies measure the tendency of a neutral atom to resist the loss of electrons. Electron affinity on the other hand is the energy released when an electron is added to the atom.
Difference Between Electron Affinity And Ionization Energy
In other words this enthalpy change and the electron affinity differ by a negative sign.
Ionization energy vs electron affinity. Ionization energy is the energy required to remove an electron away from an atom which can be written as ie. Electron affinity is the amount of energy released when an electron is added to a neutral atom or a molecule in a gaseous state to form negation ion. It generally increases across a row on the periodic maximum for the noble gases which have closed shells.
Ionization energy is the amount of energy needed to remove an electron from a neutral atom. Periodic trends atomic size ionization energy and metallic character chemistry libretexts. For example electron affinity of carbon is 153 9 kj mol.
The first ionization energy. Electron affinities are more difficult to measure than ionization energies. The ionization energy or ionization potential is the energy necessary to remove an electron from the neutral atom.
Certain properties notably atomic radius ionization energy electron affinity and metallic character can be qualitatively understood by the positions of the elements on the periodic table. In solid state physics the electron affinity for a surface is defined somewhat differently see below. On the periodic table the ie of an atom decreases as you descend from a group and increases as you go across a period also as each molecular orbital is filled or half filled.
Consequences of the relative size of ionization energies and electron affinities. An atom of carbon in the gas phase for example gives off energy when it gains an electron to form an ion of carbon. Electron affinity is the amount of energy released when electron is added to an atom.
The main difference between electron affinity and ionization energy is that electron affinity gives the amount of energy released when an atom gains an electron whereas ionization energy is the amount of energy required to remove an electron from an atom. It is a minimum for the alkali metals which have a single electron outside a closed shell. The energy needed to remove one or more electrons from a neutral atom to form apositively charged ion is a physical property that influences the chemical behavior of theatom.
Ionization energy and electron affinity. Ionization energy is the amount of energy required to remove the electron that is most loosely bound of an isolated gaseous atom to form a cation. Ionization energy is related with making cations from neutral atoms and electron affinity is related with making anions.
Note that this is not the same as the enthalpy change of electron capture ionization which is defined as negative when energy is released.
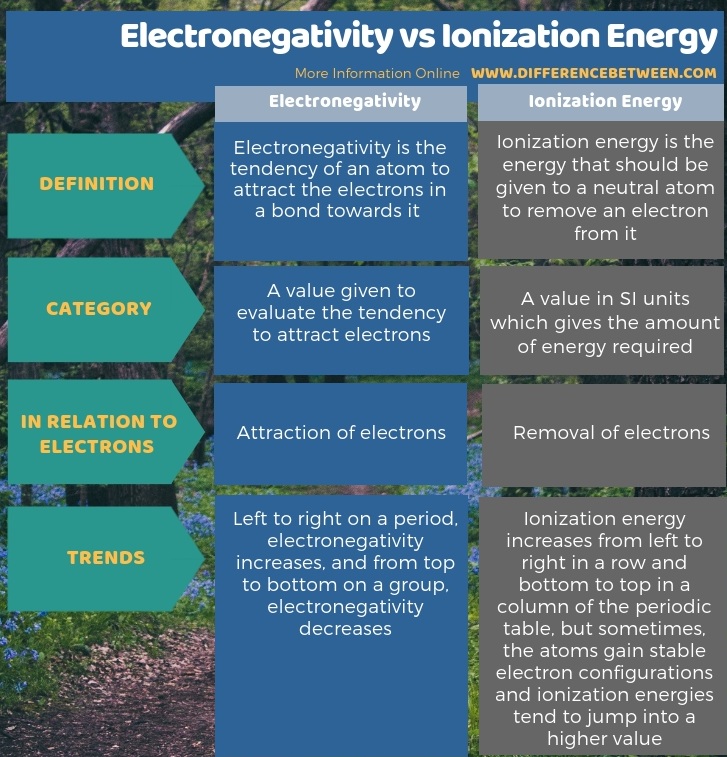 Difference Between Electronegativity And Ionization Energy
Difference Between Electronegativity And Ionization Energy
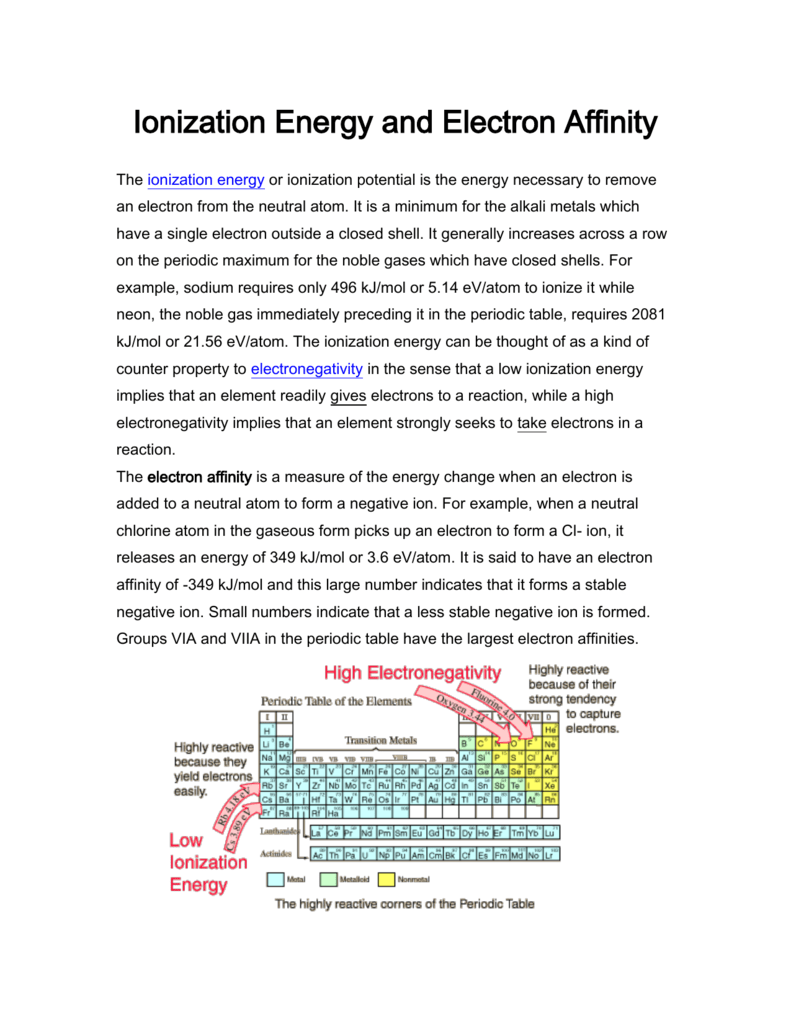 Ionization Energy And Electron Affinity
Ionization Energy And Electron Affinity
 12 Electron Affinity X Work Function W F And Ionization Energy E
12 Electron Affinity X Work Function W F And Ionization Energy E
 6 3 Periodic Trends Flashcards Quizlet
6 3 Periodic Trends Flashcards Quizlet
 Differentiate Between Ionization Energy And Electron Affinity
Differentiate Between Ionization Energy And Electron Affinity
 Ionization Energy Electron Affinity Youtube
Ionization Energy Electron Affinity Youtube
Electron Affinity Vs Ionization Energies Vs Electronegativity
 Selenium Electron Affinity Electronegativity Ionization
Selenium Electron Affinity Electronegativity Ionization
8 4 5 Ionization Energies And Electron Affinities Chemistry
 Periodic Trends Ionization Energy And Electron Affinity Youtube
Periodic Trends Ionization Energy And Electron Affinity Youtube
 How Is Electronegativity Related To Ionization Energy And Electron
How Is Electronegativity Related To Ionization Energy And Electron
Trends In The Periodic Table Course Hero
Ionization Energy And Electron Affinity
Ionization Energy And Electronegativity
 What Is The Difference Between Electron Affinity And Ionization
What Is The Difference Between Electron Affinity And Ionization
Ionization Energy And Electron Affinity
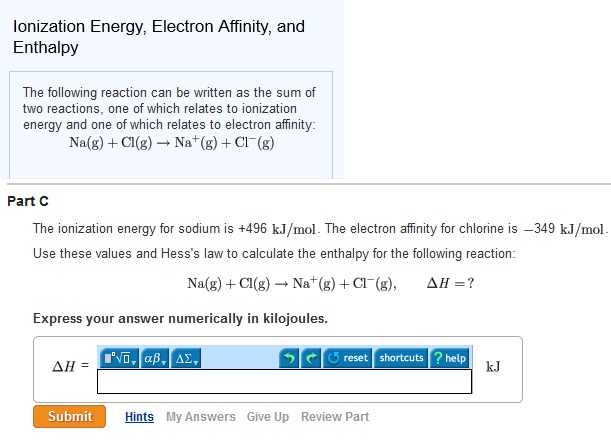 Solved Ionization Energy Electron Affinity And Enthalpy
Solved Ionization Energy Electron Affinity And Enthalpy
Difference Between Electronegativity And Electron Affinity
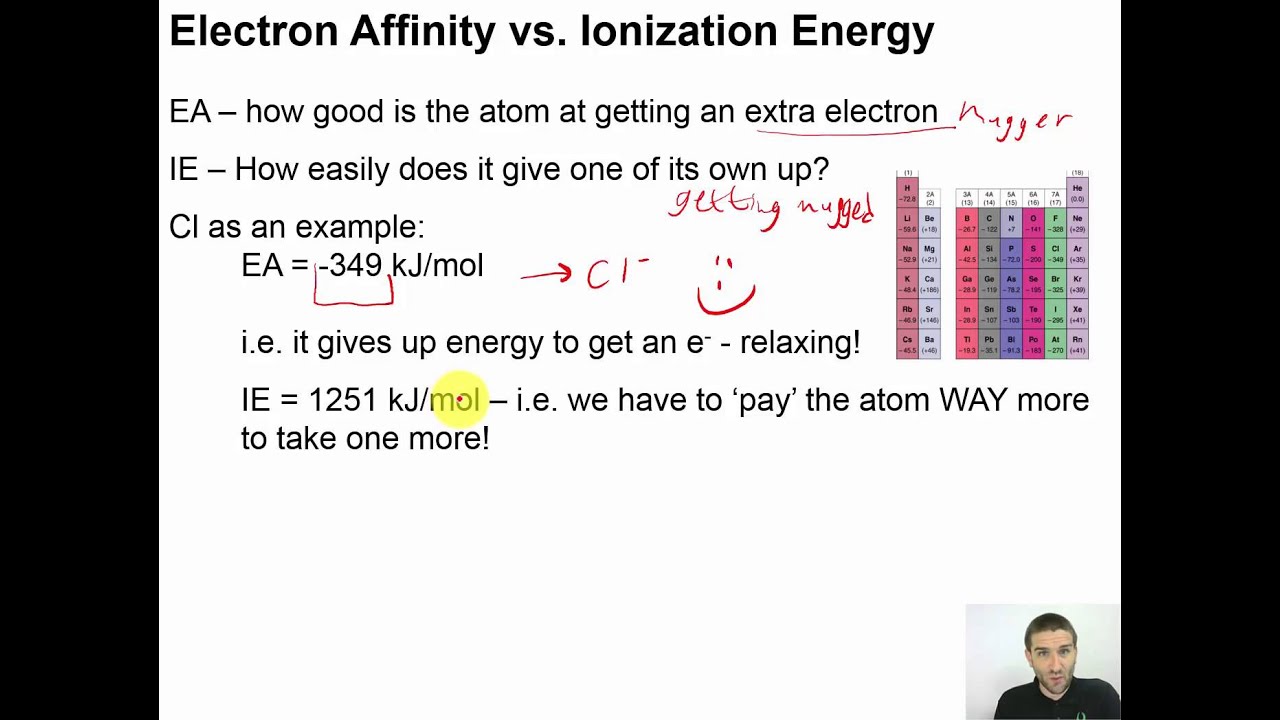 Chapter 04 23 Electron Affinity Vs Ionization Energy Youtube
Chapter 04 23 Electron Affinity Vs Ionization Energy Youtube
What Is The Difference Between Ionization Energy And Electron
 Overview Atomic Size Ionization Energy Electron Affinity Youtube
Overview Atomic Size Ionization Energy Electron Affinity Youtube
Ionization Energy And Electron Affinity
 Ionization Energy And Electron Affinity Adiabatic And Vertical
Ionization Energy And Electron Affinity Adiabatic And Vertical
 A Energy Level Diagram Showing The Ionization Energy And Electron
A Energy Level Diagram Showing The Ionization Energy And Electron
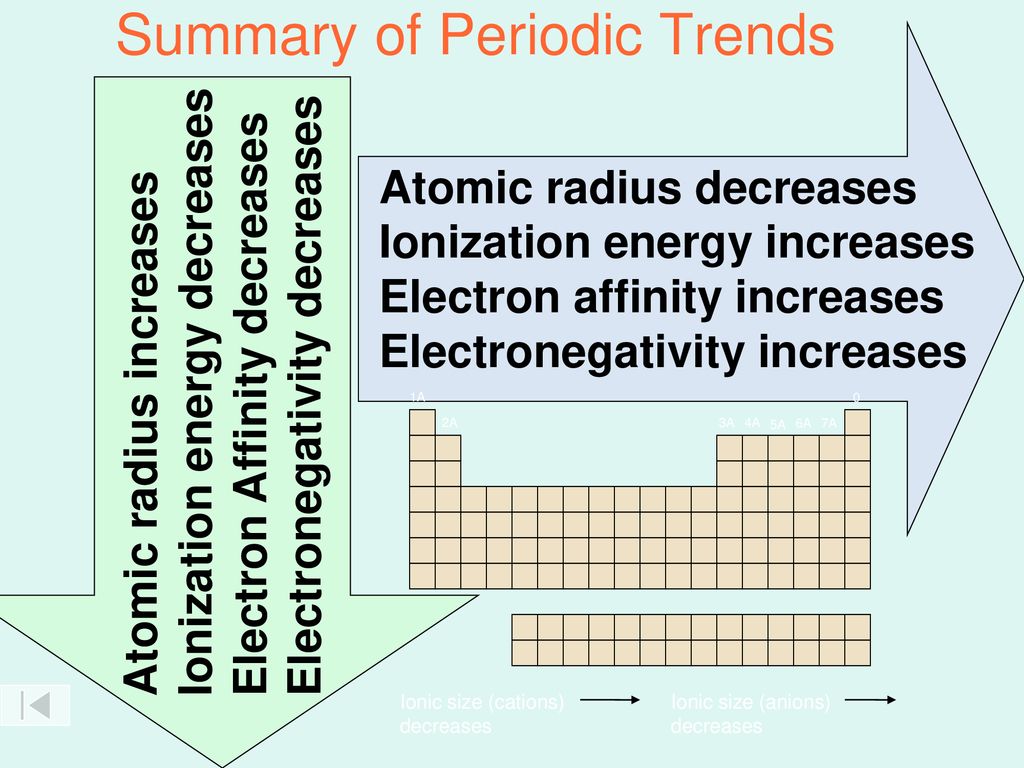 Periodic Trends Atomic Size Ionization Energy Electron Affinity
Periodic Trends Atomic Size Ionization Energy Electron Affinity
 Ionization Energy And Electron Affinity Chemistry Stack Exchange
Ionization Energy And Electron Affinity Chemistry Stack Exchange
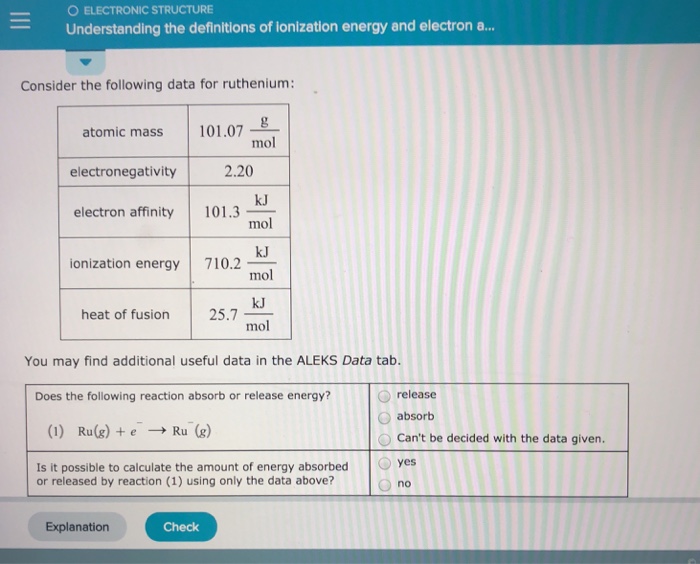
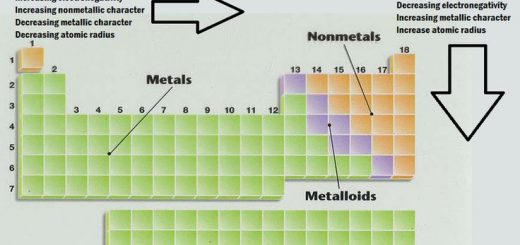 Radius Property Ionization Potential Electron Affinity
Radius Property Ionization Potential Electron Affinity
What Is The Difference Between Electron Affinity And Ionization
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctcpz999nq5hlwi7vhm8iuzdsv3x3dvmcvv9m6sl0jjguo27etp Usqp Cau
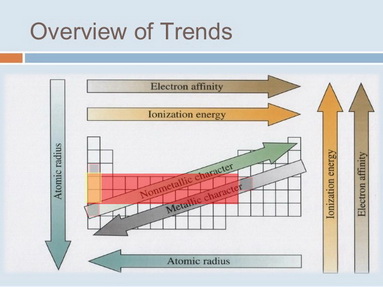 If Element X Has An Atomic Radius Larger Than Ga An Electron
If Element X Has An Atomic Radius Larger Than Ga An Electron
 High School Chemistry Electron Affinity Wikibooks Open Books
High School Chemistry Electron Affinity Wikibooks Open Books
Ionization Energy And Electronegativity
Ionization Energy And Electron Affinity

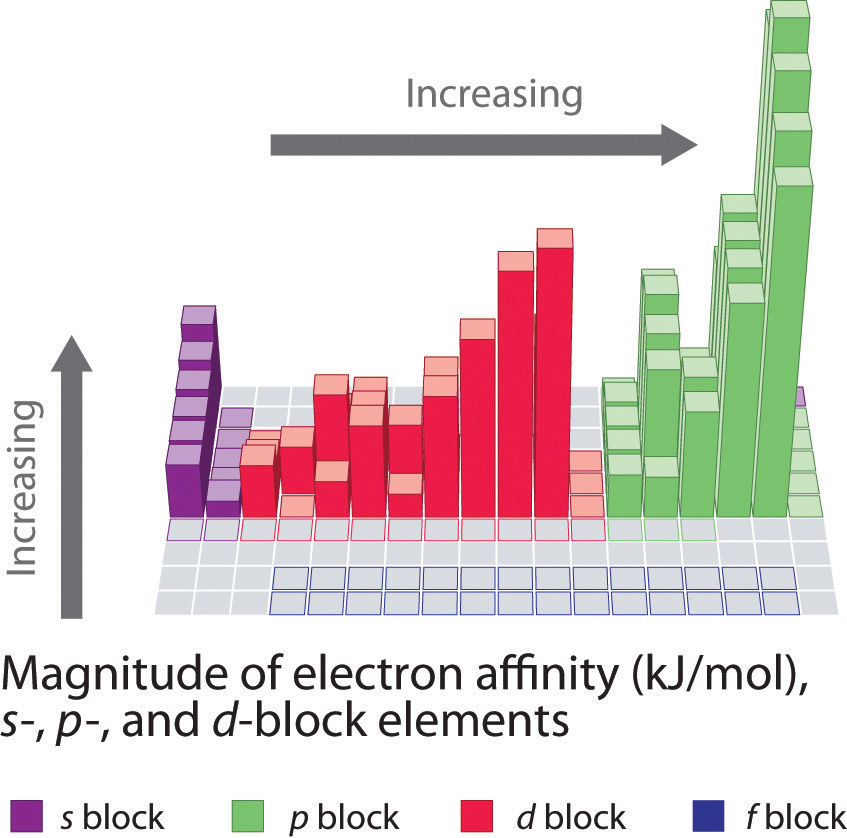
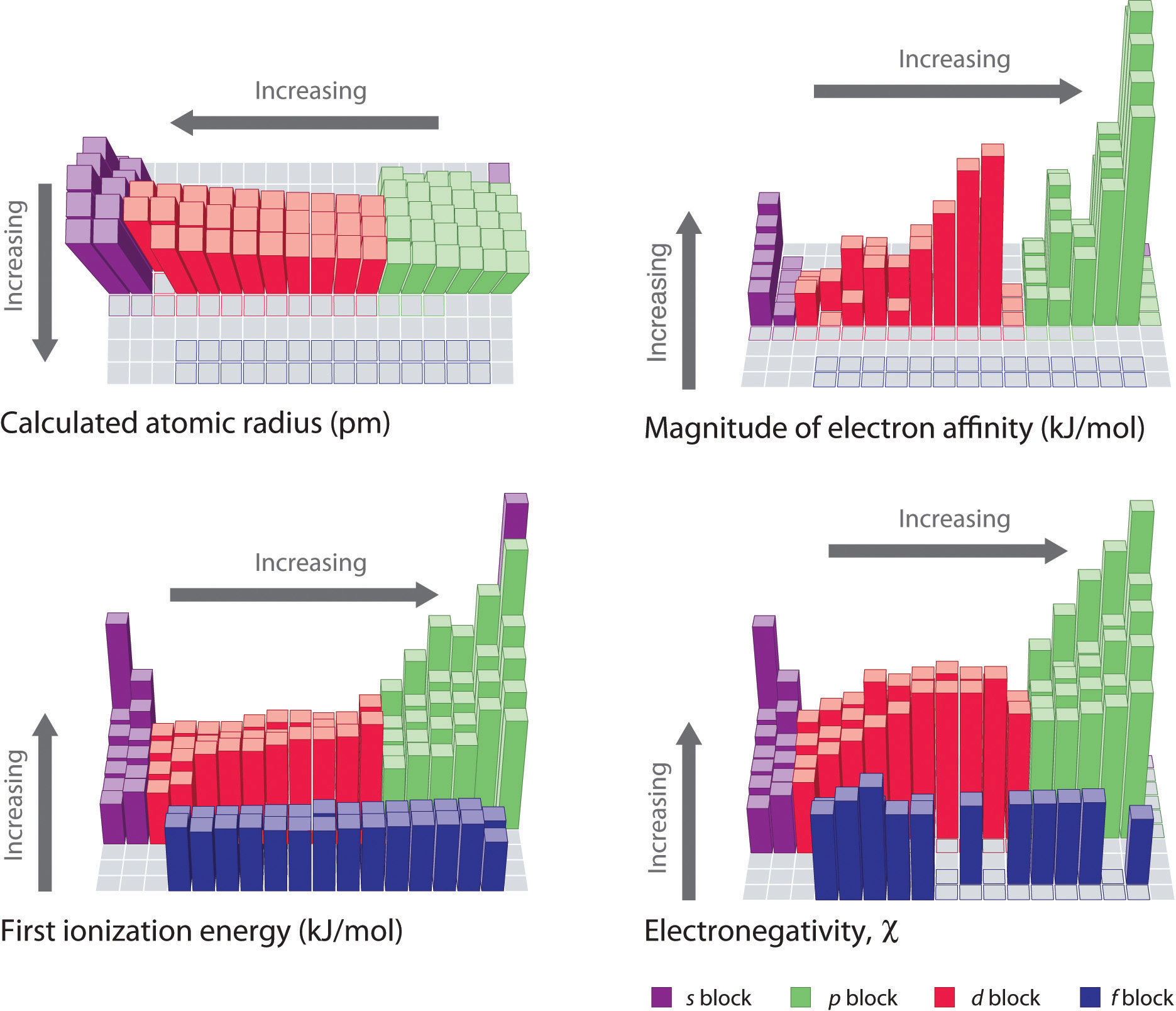
Posting Komentar
Posting Komentar