1 Mole Of Water In Grams
The molecular formula for water is h2o. For instance the molecular weight of the water molecule h 2 o is 18 02 and therefore one mole of pure water weighs 18 02 gram.
A Mole Fraction Of A In H2o Is 0 2 What Is The Molarity Of A In
The si base unit for amount of substance is the mole.

1 mole of water in grams. The gram molecular mass of water is 18 grams per mole. Weight of water 2 0158 g 15 9994 g. You can view more details on each measurement unit.
One mole of water weighs 18 015 g. The molecular formula for water is h2o. The si base unit for amount of substance is the mole.
Therefore one mole of water weighs 18 0152 grams. For water the molecular weight is 2 of hydrogen and 1 of oxygen 2x1 1x16 18 so a gram mole of water weighs 18 grams. The mass of one mole of a substance is equal to that substance s molecular weight.
If we mix 46 069 g of ethyl alcohol with 18 015 g of water we can be assured that the mixture contains 1 molecule of ethyl alcohol per molecule of water. Unless you have a good sense of mass this value probably doesn t have much meaning to you. 1 mole is equal to 1 moles water or 18 01528 grams.
Thus for example one mole of water contains 6 022 140 76 1023 molecules whose total mass is about 18 015 grams and the mean mass of one molecule of water is about 18 015 daltons. It s easier to grasp how much water is in a mole if you find the volume of this amount of mass. The answer is 18 01528.
The mole is widely used in chemistry as a convenient way to express amounts of reactants and products of chemical reactions. We assume you are converting between grams water and mole. For example the mean molecular weight of water is 18 015 atomic mass units amu so one mole of water weight 18 015 grams.
A gram mole is one gram times the molecular weight. Molecular weight of water or mol. Avogadro s number is the number of water moleculesneeded to obtain a mass of 18 grams.
This isbecause a water molecule contains two hydrogen atoms one protoneach and one oxygen atom 8 protons and 8 neutrons for a totalof 18 nucleons. 1 grams water is equal to 0 055508435061792 mole. Note that rounding errors may occur so always check the results.
The definition of a mole extends to different entities. Molecules ions atoms electrons any elementary substance in chemistry. Fortunately this is another simple calculation.
Weight of water 18 0152 g. Further we will know that there are 2 atoms of c and 8 atoms of h per each 2 atoms of o.
Https Www Unf Edu Michael Lufaso Chem2045 Chapter3 Pdf
 Chemistry Warm Up Mole Mass Particles 1 What Is The Mass Of
Chemistry Warm Up Mole Mass Particles 1 What Is The Mass Of
 How Many Grams Of Water Do You Need To Weigh If A Reaction
How Many Grams Of Water Do You Need To Weigh If A Reaction
 What Is A Mole Villanova College Chemistry Blog
What Is A Mole Villanova College Chemistry Blog
 What Is The Mass Of A 0 2 Mole Of Oxygen Atoms B 0 5 Mole Of
What Is The Mass Of A 0 2 Mole Of Oxygen Atoms B 0 5 Mole Of
 Determining An Empirical Formula From Combustion Data Worked
Determining An Empirical Formula From Combustion Data Worked
 How Many Moles Are In 25 Grams Of Water Youtube
How Many Moles Are In 25 Grams Of Water Youtube
 Mole Avogadro S Number Mass Percent Composition Ppt Video
Mole Avogadro S Number Mass Percent Composition Ppt Video
 Stoichiometry Example Problem 2 Video Khan Academy
Stoichiometry Example Problem 2 Video Khan Academy
Http Www Uh Edu Chem1p C3 C3f99 Pdf
What Is The Number Of Hydrogen Atoms In 5 Moles Of Water Quora
 Calculate The Mass Of A Single Atom Or Molecule Youtube
Calculate The Mass Of A Single Atom Or Molecule Youtube
 Entry Task Block 1 Feb 1st 2nd Block 2 Ppt Video Online Download
Entry Task Block 1 Feb 1st 2nd Block 2 Ppt Video Online Download
The Chemcollective Stoichiometry Tutorial Dimensional Analysis
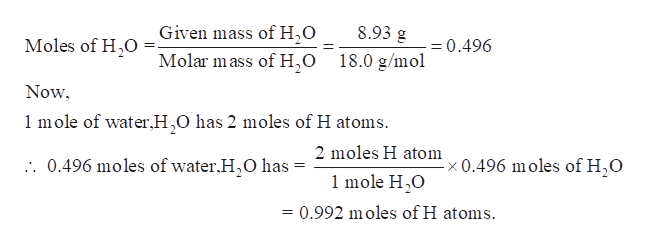 Answered How Many Atoms Of Hydrogen Are Present Bartleby
Answered How Many Atoms Of Hydrogen Are Present Bartleby
 Sample Problem 1 What Is The Mass Of Eac A 1 Mole Of Wate
Sample Problem 1 What Is The Mass Of Eac A 1 Mole Of Wate
How To Calculate The Number Of Molecules In 72 Grams Of Water Quora
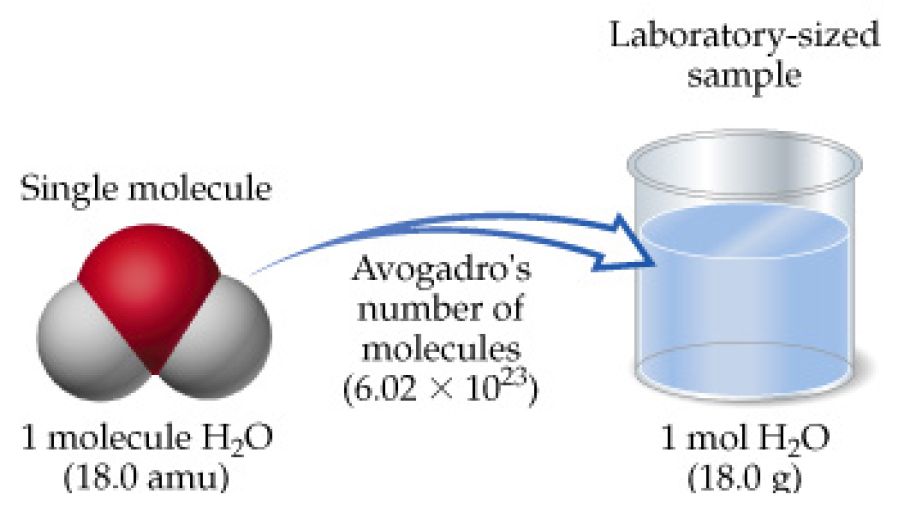
/GettyImages-692027135-419fe3ddc26e4415b356380582c4e5b2.jpg) How Much Water Is A Mole Of Water
How Much Water Is A Mole Of Water

 How Many Molecules Are In 48 90 Grams Of Water Socratic
How Many Molecules Are In 48 90 Grams Of Water Socratic
 The Mole Ch 3 3 And Ch Ppt Download
The Mole Ch 3 3 And Ch Ppt Download
 Mole Definition Number Facts Britannica
Mole Definition Number Facts Britannica
 How To Convert Moles Of H2o To Grams Youtube
How To Convert Moles Of H2o To Grams Youtube
 Science Chemistry Molarity Fundamental Photographs The Art Of
Science Chemistry Molarity Fundamental Photographs The Art Of
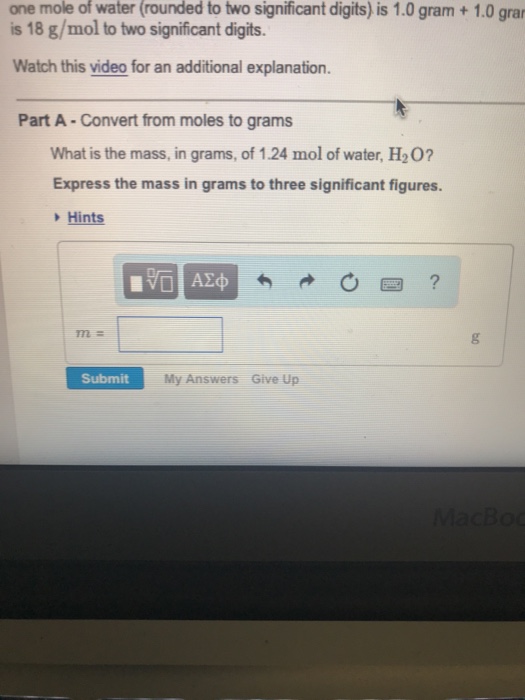
How Many Atoms Are Present In One Mole Of Water Quora
 How Many Moles Of Water Will Be Produced When 8 Grams Of Hydrogen
How Many Moles Of Water Will Be Produced When 8 Grams Of Hydrogen

 Mole Avogadro S Number Mass Percent Composition Ppt Video
Mole Avogadro S Number Mass Percent Composition Ppt Video
 1 5 Mole Equations Easy Fast Learn
1 5 Mole Equations Easy Fast Learn
 Chemistry Warm Up Mole Mass Particles 1 What Is The Mass Of
Chemistry Warm Up Mole Mass Particles 1 What Is The Mass Of
 How To Calculate The Number Of Moles Of Hydrogen Atoms Socratic
How To Calculate The Number Of Moles Of Hydrogen Atoms Socratic
 Atomic Mass And The Mole Topic Amu S Atomic Mass Objectives
Atomic Mass And The Mole Topic Amu S Atomic Mass Objectives
 Lesson 3 4 Avogadro S Number And The Mole
Lesson 3 4 Avogadro S Number And The Mole
 Number Of Atoms And Molecules Present In One Water Drop
Number Of Atoms And Molecules Present In One Water Drop
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcs 5rwgjud Xpwbiissqp9ufw14rapy1v3d73u9aw7x3v0bwe3e Usqp Cau
/what-is-a-mole-and-why-are-moles-used-602108-FINAL-CS-01-5b7583f6c9e77c00251d4d68.png)
Posting Komentar
Posting Komentar