Heat Of Reaction Definition
In thermodynamics heat is energy in transfer to or from a thermodynamic system by mechanisms other than thermodynamic work or transfer of matter. Define heat of reaction.
 1 Thermochemistry Chapter 5 No Calculators 2 Both Of These
1 Thermochemistry Chapter 5 No Calculators 2 Both Of These
The heat of reaction also known and enthalpy of reaction is the change in the enthalpy of a chemical reaction that occurs at a constant pressure.

Heat of reaction definition. Heat of reaction the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the same temperature. The enthalpy of reaction δh rxn is the difference between the total enthalpy of the products of a reaction and the total enthalpy of the reactants. If the pressure in the vessel containing the reacting system is kept at a constant value the measured heat of reaction also represents the change in the thermodynamic quantity called enthalpy or heat content accompanying the.
It is a thermodynamic unit of measurement useful for calculating the amount of energy per mole either released or produced in a reaction. N chem the heat evolved or absorbed when one mole of a product is formed at constant pressure. Heat of reaction definition is the heat evolved or absorbed during a chemical reaction taking place under conditions of constant temperature and of either constant volume or more often constant pressure.
Heat of reaction synonyms heat of reaction pronunciation heat of reaction translation english dictionary definition of heat of reaction. Calculating enthalpy changes using hess s law enthalpy definition in chemistry and physics. Look it up now.
The heat evolved or absorbed when one mole of a product is formed at constant pressure meaning pronunciation translations and examples. Like thermodynamic work heat transfer is a process involving more than one system not a property of any one system. Reaction definition is the act or process or an instance of reacting.
How to use reaction in a sentence. The various mechanisms of energy transfer that define heat are stated in the next section of this article. The quantity involved when gram equivalents of the substances enter into the reaction.
Heat of reaction definition.
 Heat Of Reaction Is Defined As The Amount Of Heat Absorbed Or Evol
Heat Of Reaction Is Defined As The Amount Of Heat Absorbed Or Evol
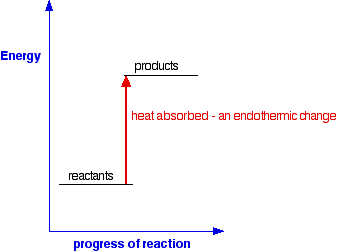 Chemical Energetics An Introduction
Chemical Energetics An Introduction
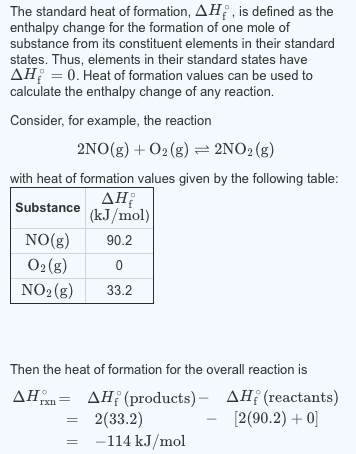 Solved The Standard Heat Of Formation Is Defined As Th
Solved The Standard Heat Of Formation Is Defined As Th
 Heat Of Reaction Measure Reaction Enthalpy Of Chemical Reactions
Heat Of Reaction Measure Reaction Enthalpy Of Chemical Reactions
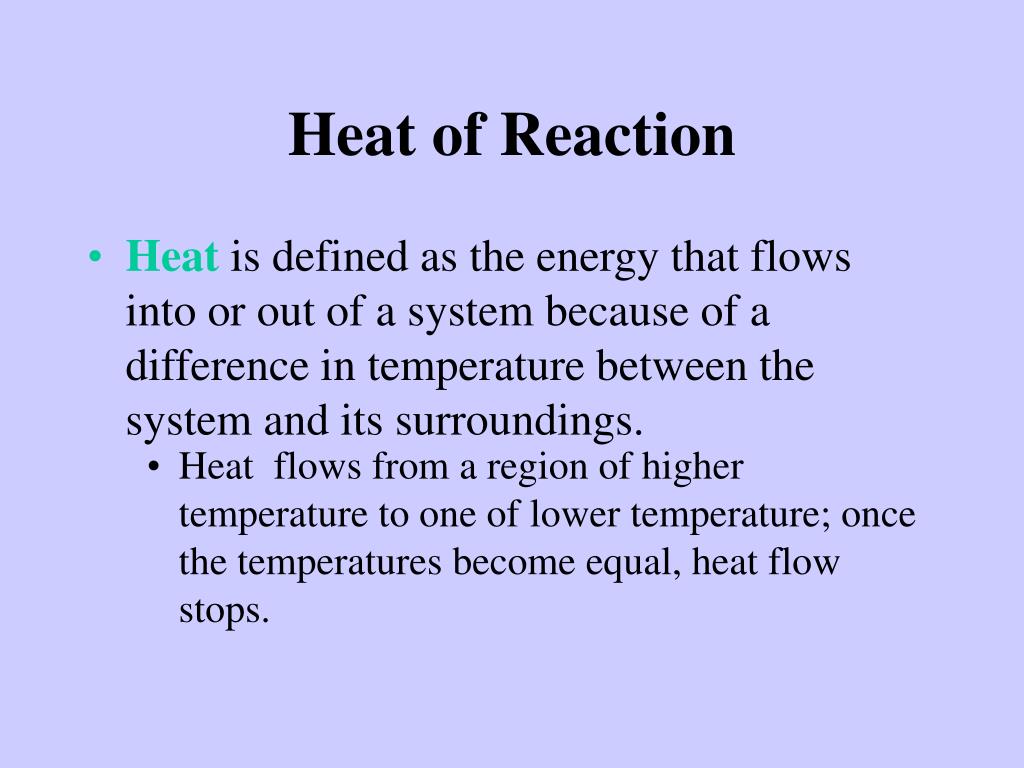 Ppt Thermochemistry Powerpoint Presentation Free Download Id
Ppt Thermochemistry Powerpoint Presentation Free Download Id
 Difference Between Heat Of Formation And Heat Of Reaction
Difference Between Heat Of Formation And Heat Of Reaction
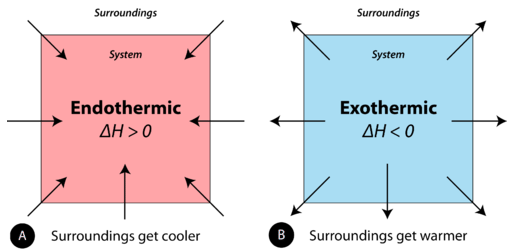 8 7 Enthalpy Change Is A Measure Of The Heat Evolved Or Absorbed
8 7 Enthalpy Change Is A Measure Of The Heat Evolved Or Absorbed
 Heat Of Formation Video Enthalpy Khan Academy
Heat Of Formation Video Enthalpy Khan Academy
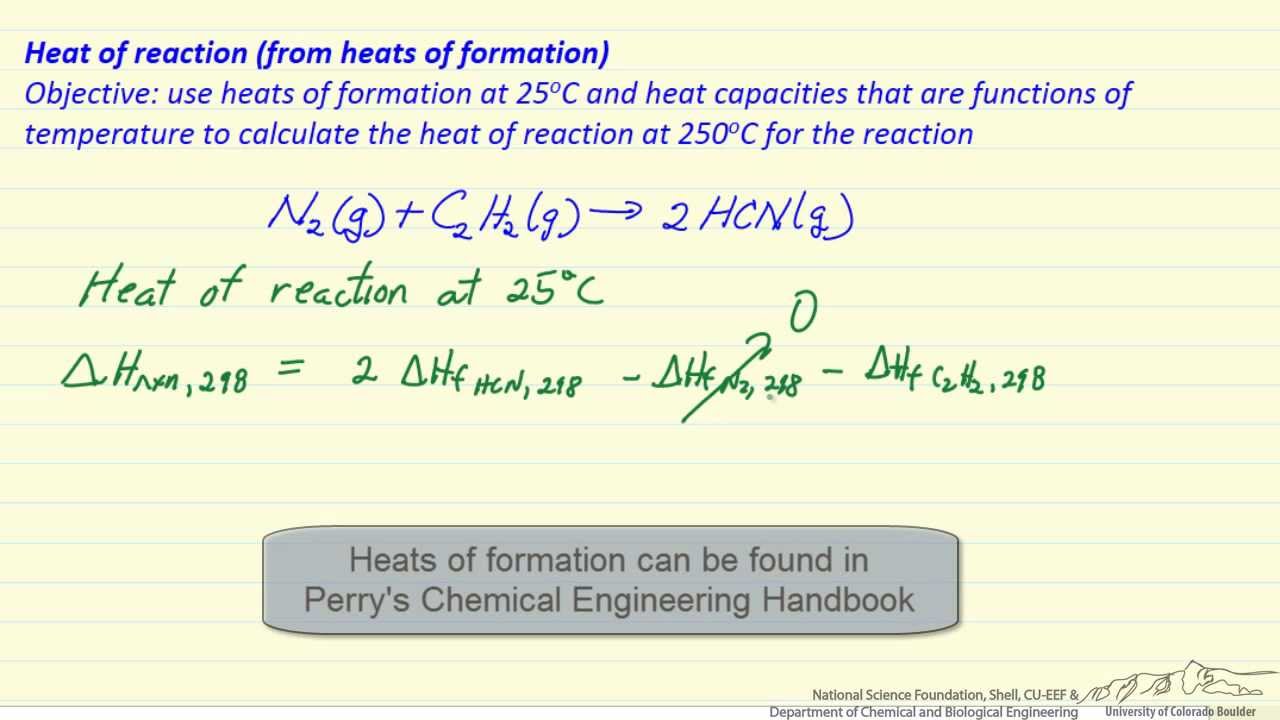 Heat Of Reaction From Heat Of Formation Youtube
Heat Of Reaction From Heat Of Formation Youtube
 Heat Of Reaction Chemistry Britannica
Heat Of Reaction Chemistry Britannica
 Chemical Reactions That Involve Heat
Chemical Reactions That Involve Heat
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcqbuyfcomsntyhhkisfdaeawtegd7w0wjg5zliiafdawaigy0u1 Usqp Cau

 Heat Of Reaction Example Youtube
Heat Of Reaction Example Youtube
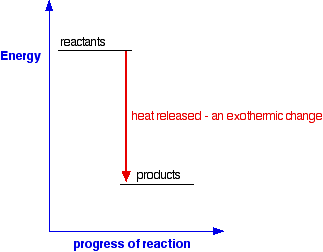 Chemical Energetics An Introduction
Chemical Energetics An Introduction

 3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow
3 Ways To Calculate The Enthalpy Of A Chemical Reaction Wikihow
 Thermochemistry 6 1 Energy And Its Units 6 2 Heat Of Reaction 6 3
Thermochemistry 6 1 Energy And Its Units 6 2 Heat Of Reaction 6 3
Difference Between Heat Of Formation And Heat Of Reaction
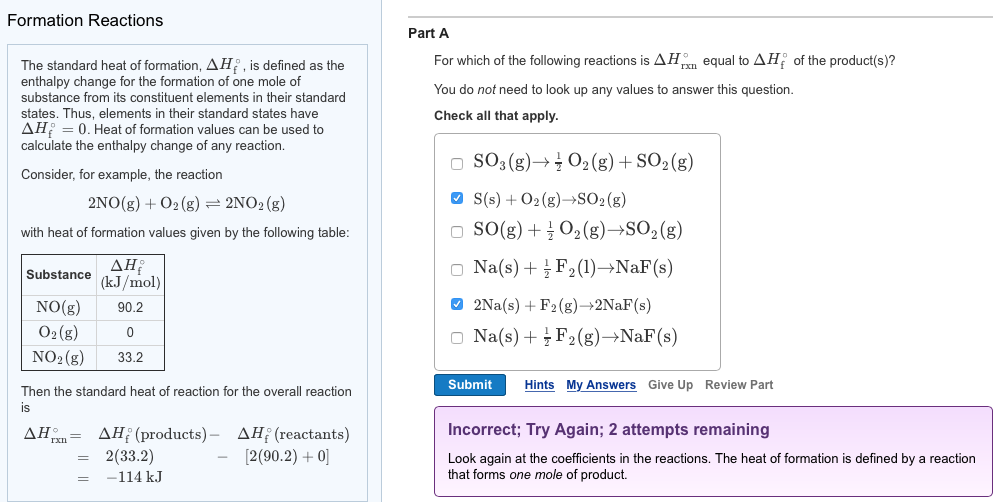 Solved The Standard Heat Of Formation Delta H Degree F
Solved The Standard Heat Of Formation Delta H Degree F
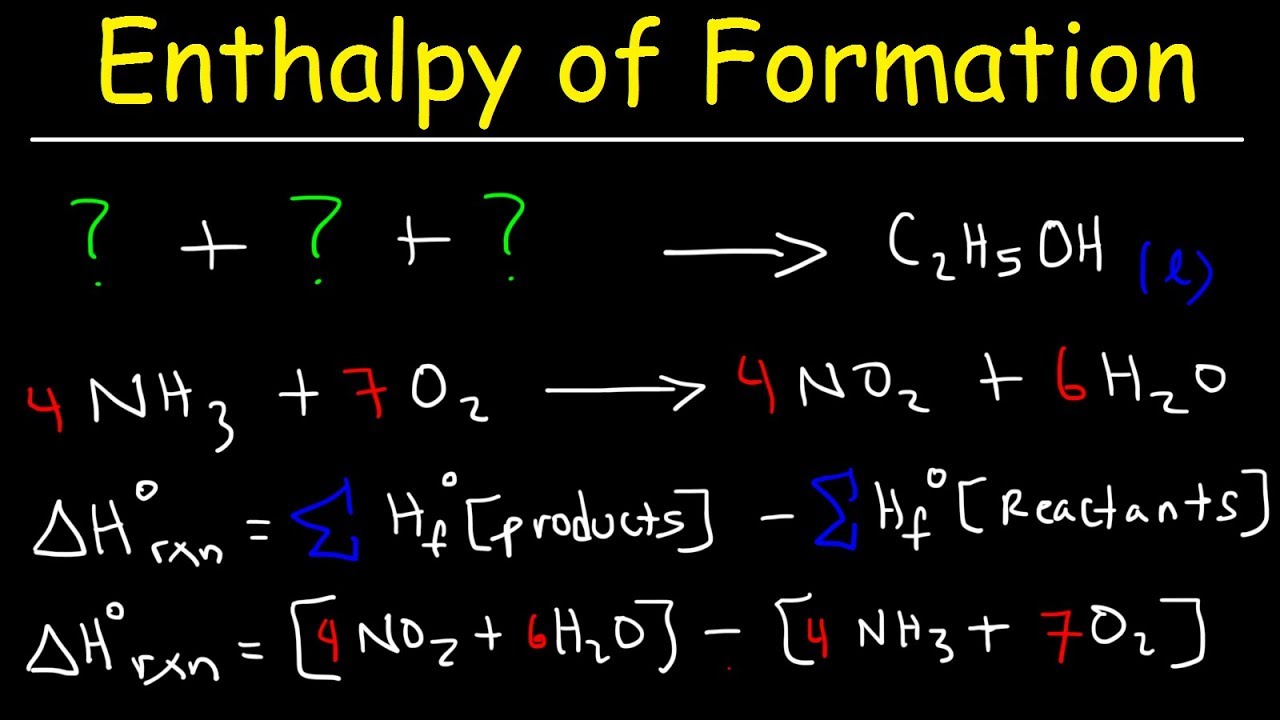 Enthalpy Of Formation Reaction Heat Of Combustion Enthalpy
Enthalpy Of Formation Reaction Heat Of Combustion Enthalpy
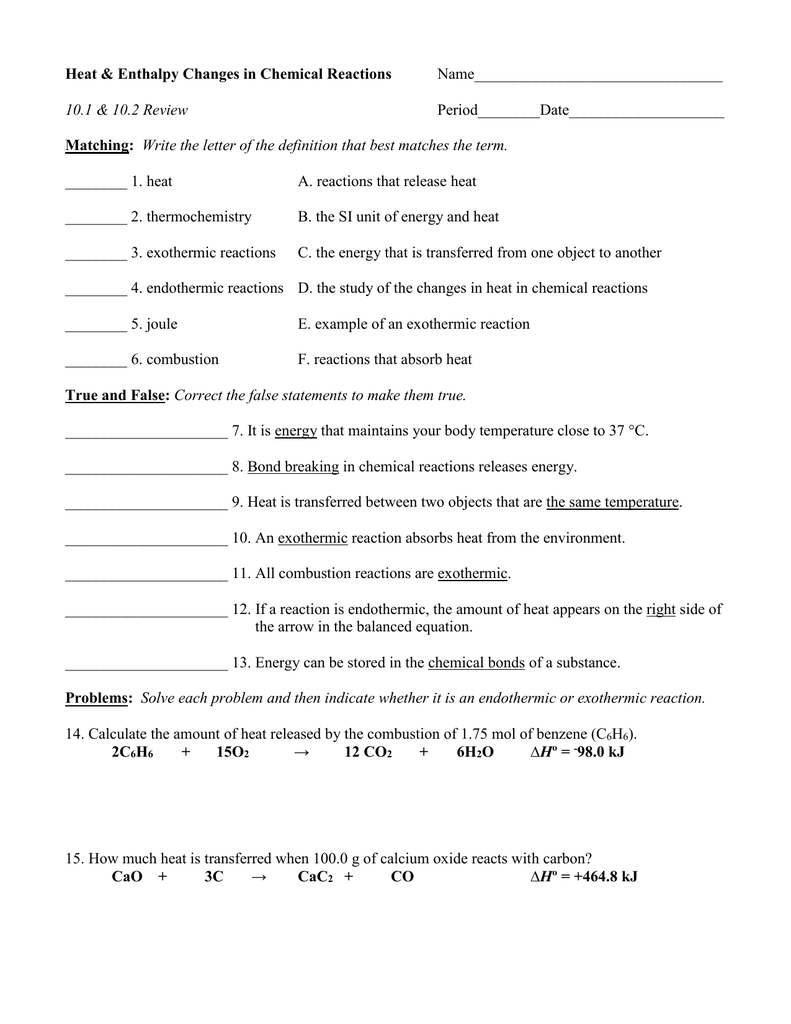 Heat Amp Enthalpy Changes In Chemical Reactions Matching
Heat Amp Enthalpy Changes In Chemical Reactions Matching
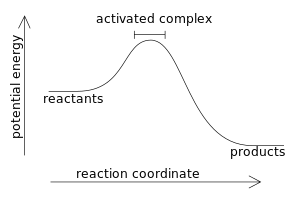
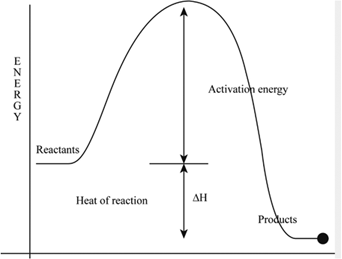 Definition Of Activation Energy Chegg Com
Definition Of Activation Energy Chegg Com
/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png) Endothermic And Exothermic Chemical Reactions
Endothermic And Exothermic Chemical Reactions
 Purpose Of The Experiment Thermochemistry Heat Of Reaction
Purpose Of The Experiment Thermochemistry Heat Of Reaction
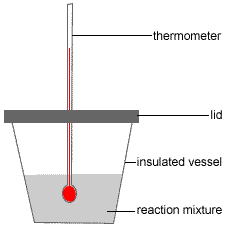 Heat Of Reaction Chemistry Tutorial
Heat Of Reaction Chemistry Tutorial

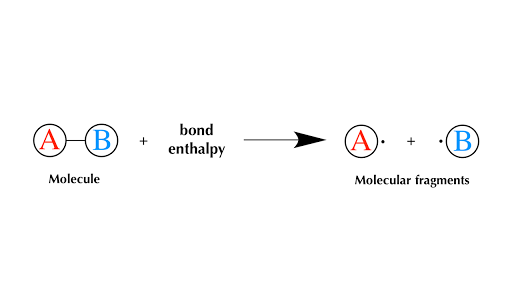
Heat Of Reaction Or Enthalpy Of Reaction Qs Study
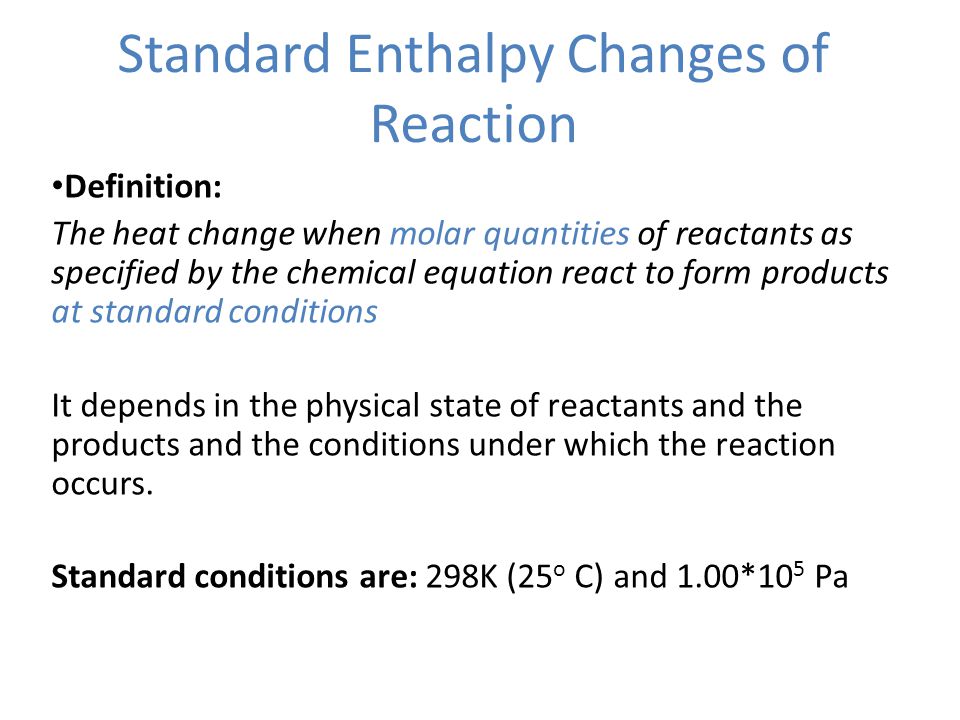 Chapter 15 Standard Enthalpy Change Of A Reaction Ppt Video
Chapter 15 Standard Enthalpy Change Of A Reaction Ppt Video

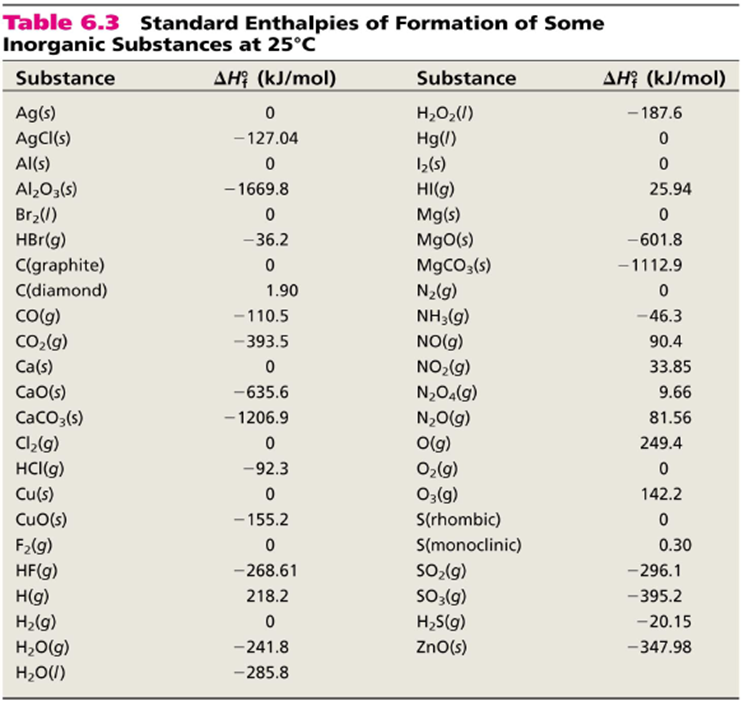

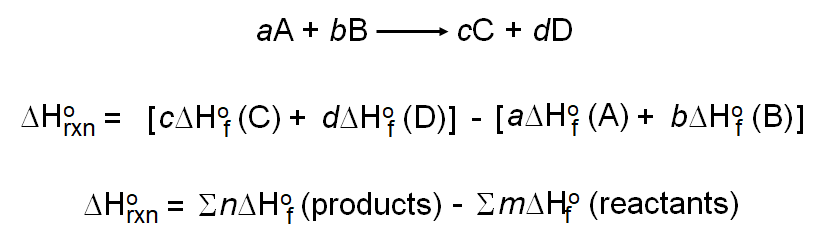


Posting Komentar
Posting Komentar