What Are Resonance Structures And Why Do We Use Them
It is as if a lone pair drops down to form another bond pushing a bond off to form a lone pair. Write the complete and balanced chemical equation.
 Drawing Lewis Structures Resonance Structures Chemistry
Drawing Lewis Structures Resonance Structures Chemistry
Show transcribed image text.
What are resonance structures and why do we use them. In such cases resonance structures are used to describe chemical bonding. In chemistry resonance is a way of describing bonding in certain molecules or ions by the combination of several contributing structures or forms also variously known as resonance structures or canonical structures into a resonance hybrid or hybrid structure in valence bond theory it has particular value for describing delocalized electrons within certain molecules or polyatomic ions. Resonance is defined by oxford dictionary as the condition in which an object or system is subjected to an oscillating force having a frequency close to its own natural frequency what is a natural frequency.
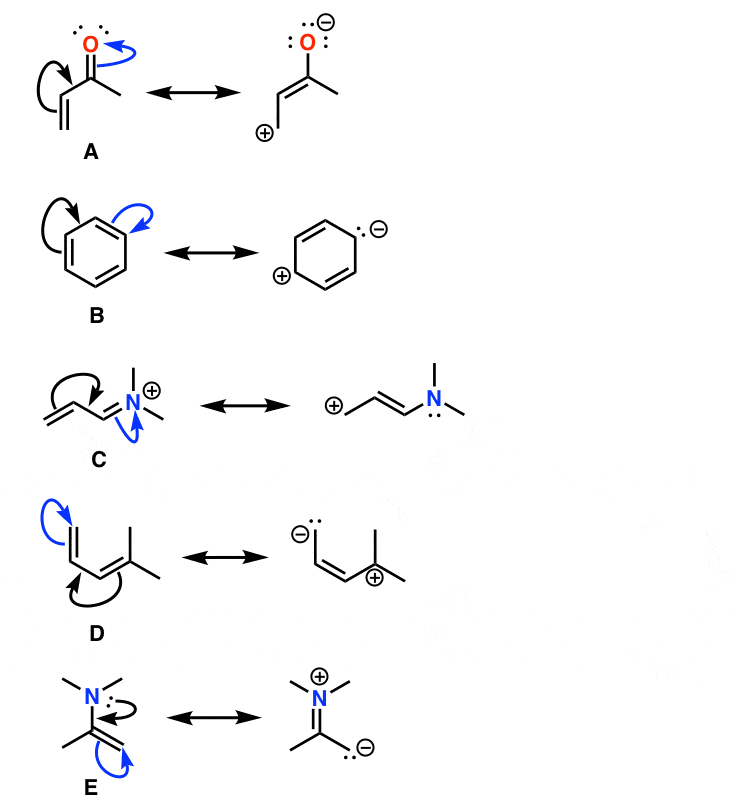
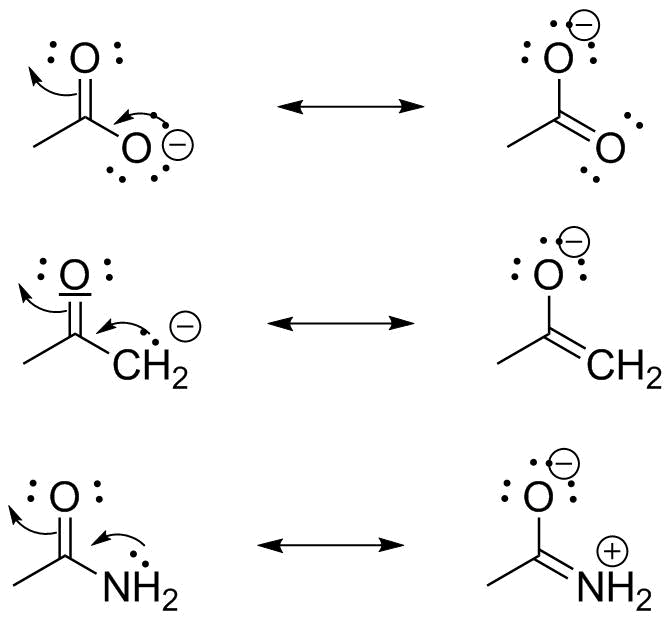
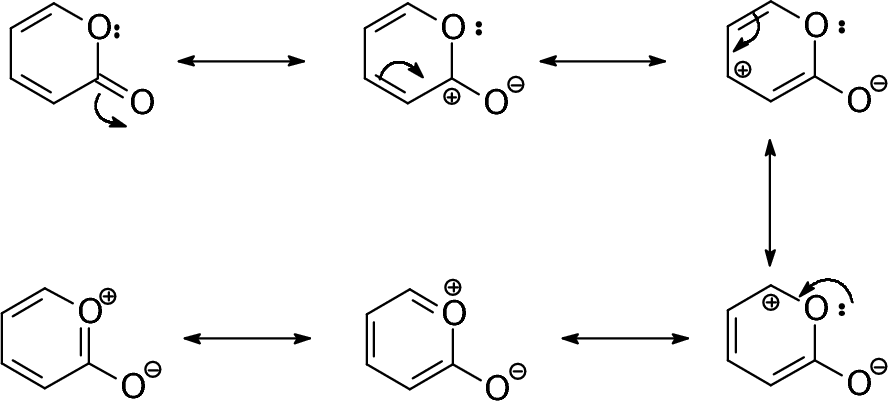
To compose the resonance structures we imagine the electron pairs shifting as shown by the small arrows below. What are resonance structures and why do we use them. Magnesium carbonate decomposes to magnesium oxide and carbon dioxide.
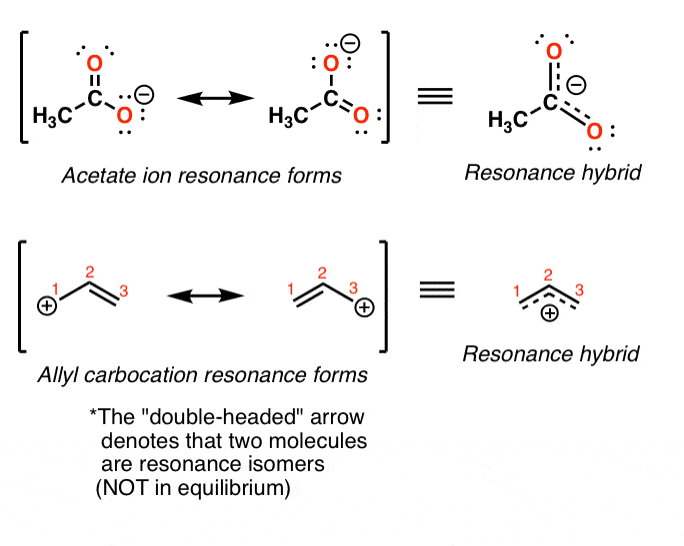
Resonance structures are separated by a double headed arrow. Notice again that only the arrangement of electrons is different in resonance structures atoms have the same connectivity. We just find it is useful to think of resonance structures in this way.
In many cases a single lewis structure fails to explain the bonding in a molecule polyatomic ion due to the presence of partial charges and fractional bonds in it. Do not use two arrows as they are used for equilibrium reactions. Remember that we do not believe this is really happening.
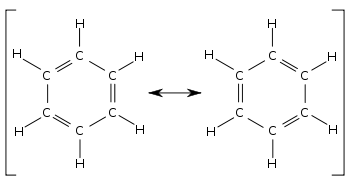
Every structure from a simple wine glass to a 100 story skyscraper has a natural frequency. These two structures are called resonance structures or resonance forms of the same compound. This problem has been solved.
Resonance structures are sets of lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule.
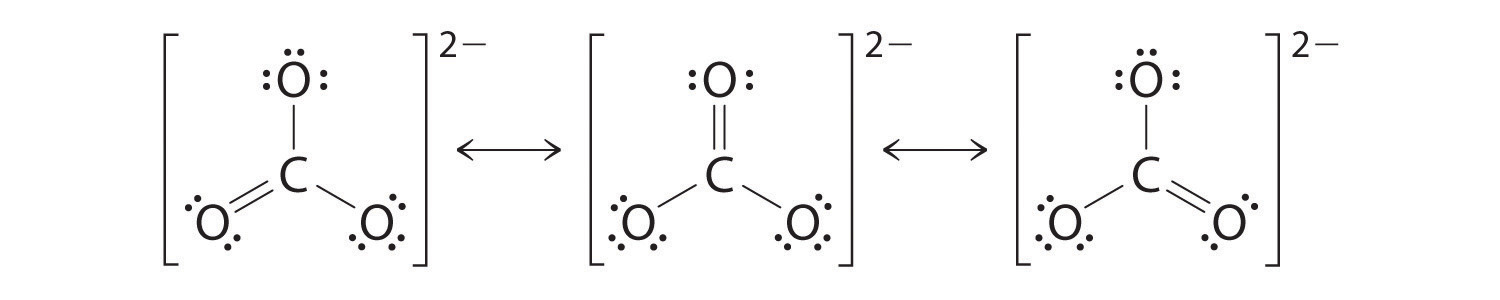 10 5 Resonance Chemistry Libretexts
10 5 Resonance Chemistry Libretexts
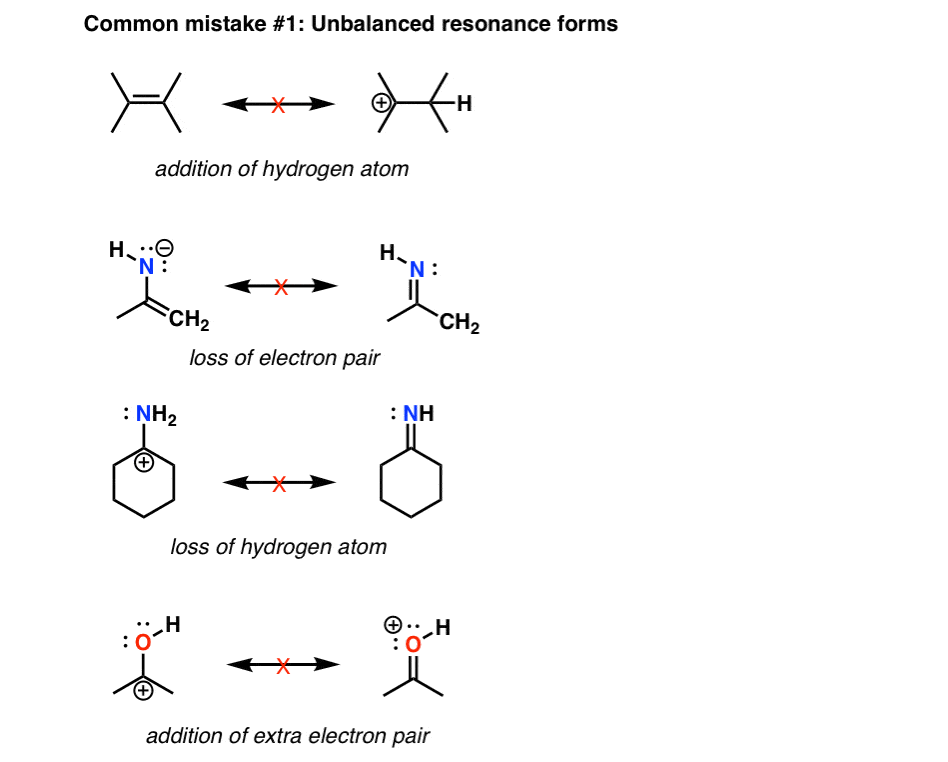 Drawing Resonance Structures 3 Common Mistakes To Avoid
Drawing Resonance Structures 3 Common Mistakes To Avoid
 Why Can One Double Bond Exist In Some Resonance Structures But Two
Why Can One Double Bond Exist In Some Resonance Structures But Two
In Chemistry What Are Resonance Structures Quora
 6 2 Resonance Organic Chemistry 1 An Open Textbook
6 2 Resonance Organic Chemistry 1 An Open Textbook
 Resonance Structures In Organic Chemistry With Practice Problems
Resonance Structures In Organic Chemistry With Practice Problems
 Which Of The Resonance Structures Is More Stable Chemistry
Which Of The Resonance Structures Is More Stable Chemistry
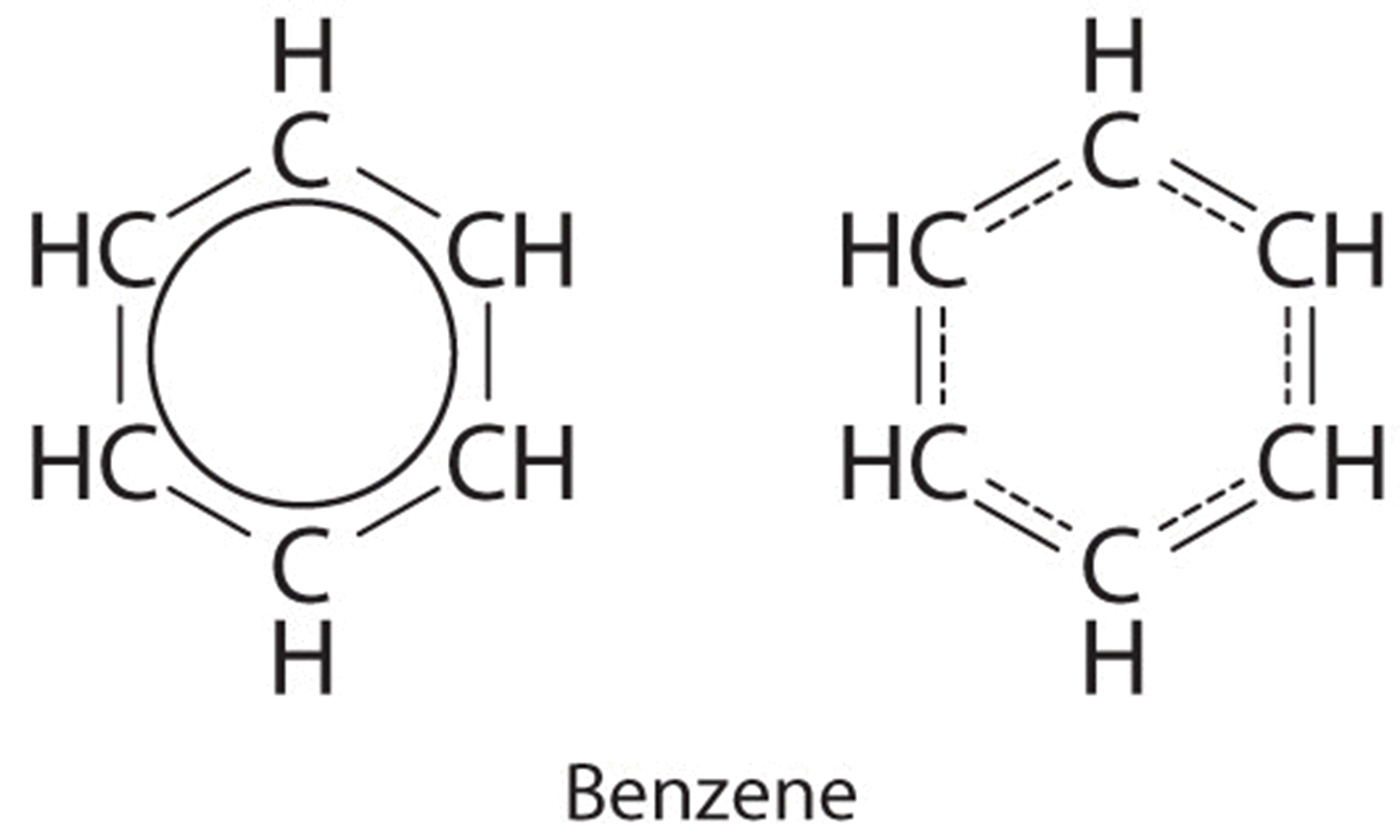 8 6 Resonance Structures Chemistry Libretexts
8 6 Resonance Structures Chemistry Libretexts
How To Move Electrons To Transform One Resonance Structure Into
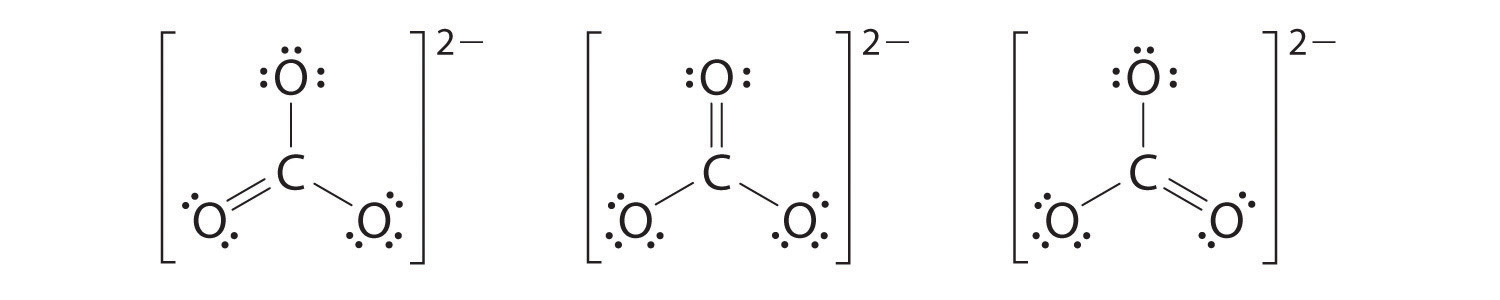
How Would You Draw All The Resonance Structures For Nitrate No3
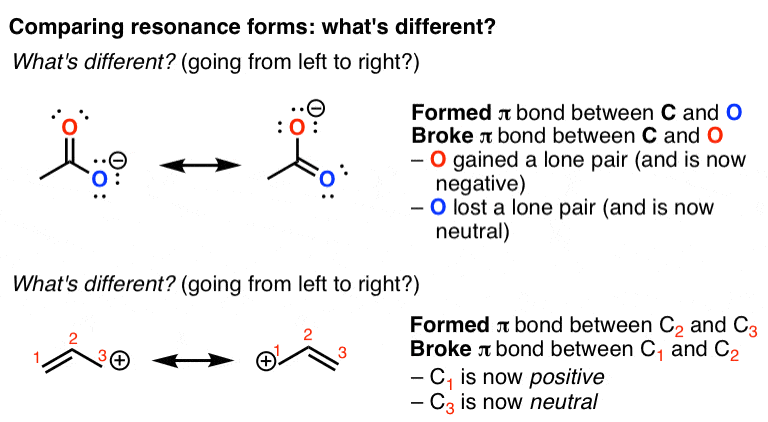 How To Use Curved Arrows To Interconvert Resonance Forms
How To Use Curved Arrows To Interconvert Resonance Forms
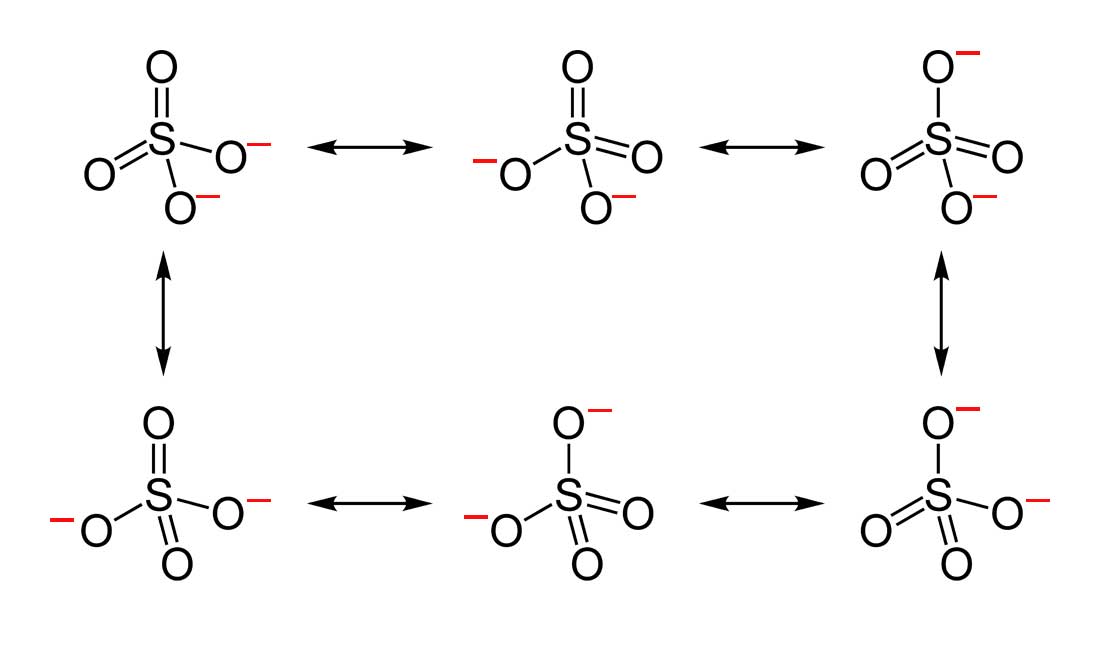
 Which Resonance Structure Of Sulfur Trioxide Represents Its True
Which Resonance Structure Of Sulfur Trioxide Represents Its True
 Resonance Structures 4 Rules On How To Evaluate Them With Practice
Resonance Structures 4 Rules On How To Evaluate Them With Practice
How To Write Resonance Structures Quora
 Drawing Resonance Structures 3 Common Mistakes To Avoid
Drawing Resonance Structures 3 Common Mistakes To Avoid
 What Are The Correct Resonance Structures Of Bromoethene
What Are The Correct Resonance Structures Of Bromoethene
How To Assign Major And Minor Resonance Structures
How Do Resonance Structures And Isomers Differ Socratic
 Resonance Chemistry Libretexts
Resonance Chemistry Libretexts
 8 6 Resonance Structures Chemistry Libretexts
8 6 Resonance Structures Chemistry Libretexts
 Resonance Structures Of Amide A4 Uracil Derivatives And Their
Resonance Structures Of Amide A4 Uracil Derivatives And Their
 How To Choose The More Stable Resonance Structure Chemistry Steps
How To Choose The More Stable Resonance Structure Chemistry Steps
 6 2 Resonance Organic Chemistry 1 An Open Textbook
6 2 Resonance Organic Chemistry 1 An Open Textbook
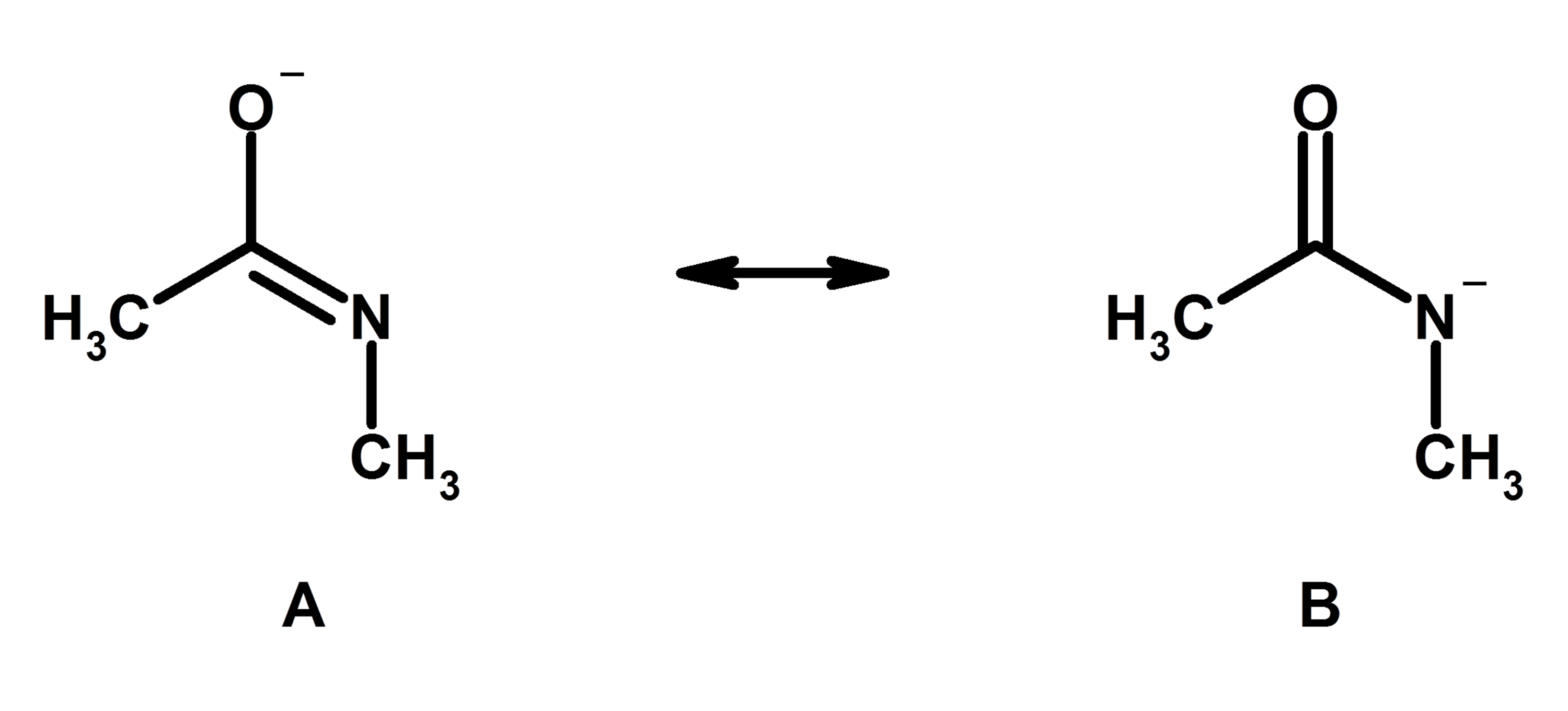 What Is Resonance 7 Rules To Master It Free Guide Organic
What Is Resonance 7 Rules To Master It Free Guide Organic
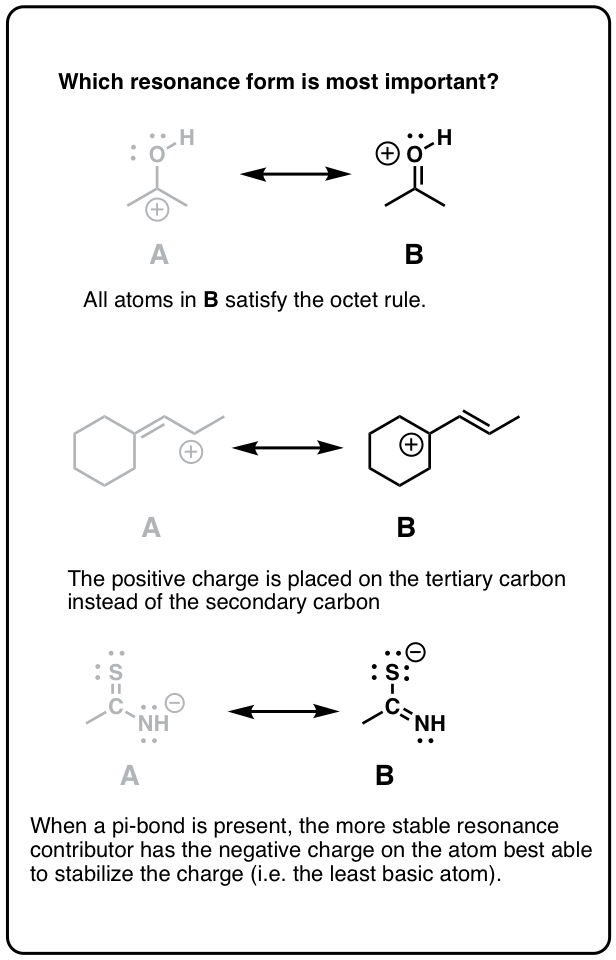 Resonance Structures 4 Rules On How To Evaluate Them With Practice
Resonance Structures 4 Rules On How To Evaluate Them With Practice
 How Are These Two Organic Resonance Structures Different
How Are These Two Organic Resonance Structures Different
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcqkhuhh3ie6dpl1bzitqmkdhgetad4plnuhye6ja4g Fszcni7f Usqp Cau
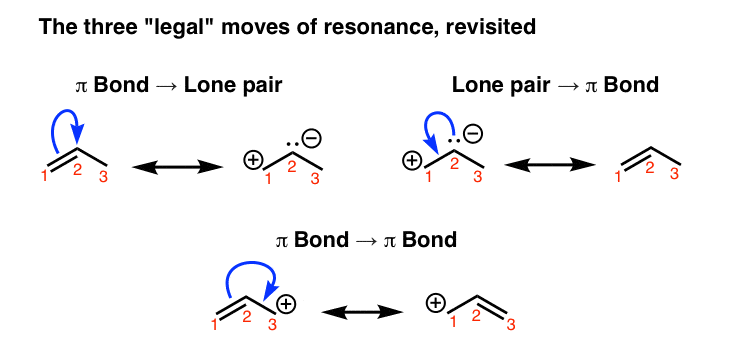 Resonance Structures 4 Rules On How To Evaluate Them With Practice
Resonance Structures 4 Rules On How To Evaluate Them With Practice
 The Predicted Stabilities Of Resonance Contributors Mcc Organic
The Predicted Stabilities Of Resonance Contributors Mcc Organic
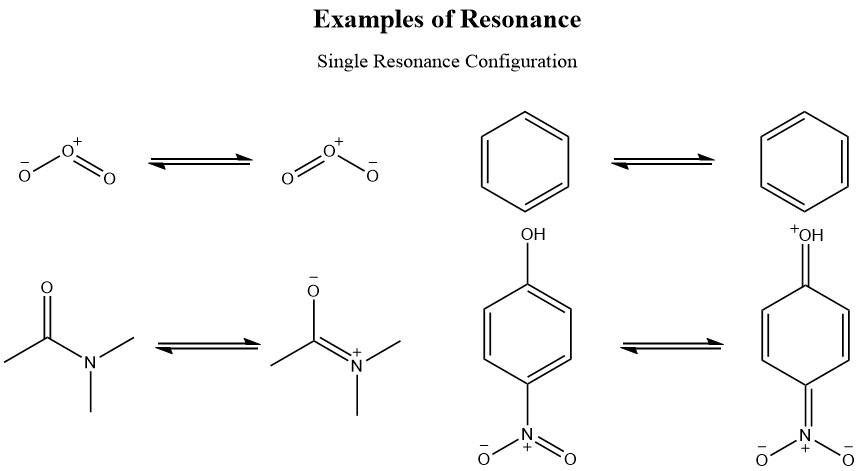 2 5 Rules For Resonance Forms Chemistry Libretexts
2 5 Rules For Resonance Forms Chemistry Libretexts


Posting Komentar
Posting Komentar