What Are Resonance Structures
The net sum of valid resonance structures is defined as a resonance hybrid which represents the overall delocalization of electrons within the molecule. We need to draw another resonance structure.
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcqgp5edzzanhyakllozaf4p3kq1oiawtkn1pydta0bxs1qgbalr Usqp Cau
In reality the positive charge of this molecule is spread out across carbons as the hybrid shows rather than concentrated on any one of the.

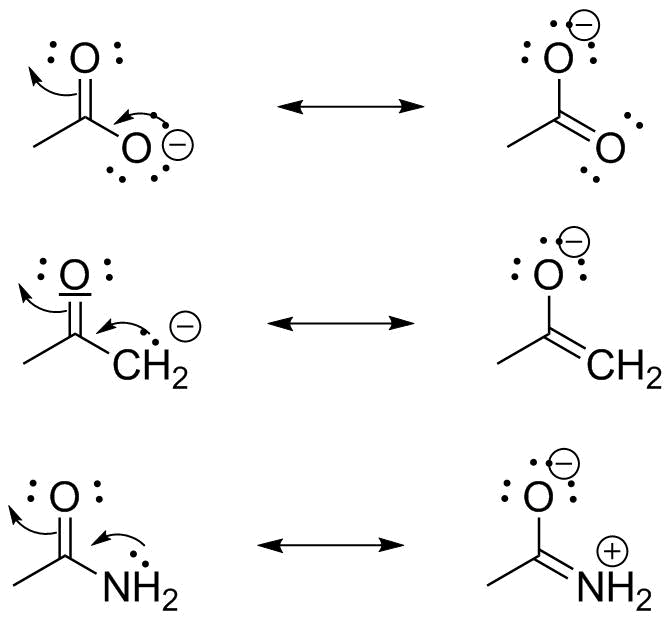
What are resonance structures. One good pattern to remember is that resonance structures involve a π bond one way or the other. In lewis structure of no 3 ion there are three lone pairs in the last shell in two oxygen atom and that oxygen atoms. This is the acetate anion and this dot structure does not completely describe the acetate anion.
Therefore whenever asked to draw a resonance structure s look for a π bond. In chemistry resonance is a way of describing bonding in certain molecules or ions by the combination of several contributing structures or forms also variously known as resonance structures or canonical structures into a resonance hybrid or hybrid structure in valence bond theory it has particular value for describing delocalized electrons within certain molecules or polyatomic ions. Some resonance structures are more favorable than others.
Resonance structures are sets of lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule. Resonance structures organic chemistry resonance organic chemistry resonance structures goc iit jee general organic chemistry for iit jee general organic chemistry class 11 general organic. When you are drawing resonance structures it is important to remember to shift only the electrons.
It is either making a bond or breaking a bond or both. Resonance structures of no 3 ion. Resonance structures and the resonance hybrid.
Here is an example of 2 resonance structures. In many cases a single lewis structure fails to explain the bonding in a molecule polyatomic ion due to the presence of partial charges and fractional bonds in it. Sometimes one dot structures is not enough to completely describe a molecule or an ion sometimes you need two or more and here s an example.
Resonance is possible whenever a lewis structure has a multiple bond and an adjacent atom with at least one lone pair. Resonance structures of the nitrate ion the nitrate ion has three valid contributing structures that vary according to the placement of the electrons. Lone pairs charges and bonds of no 3 ion.
When we draw resonance structures we convert lone pairs to bonds and bonds to lone pairs when it is possible. The arrows show how you can think of the electrons shifting as one resonance structure changes to another. You can t have resonance structures with having a π bond involved.
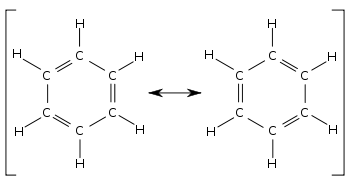
A molecule that has several resonance structures is more stable than one with fewer. The following is the general form for resonance in a structure of this type. The atoms must have the same position.
Resonance structures then are the various concrete structures that contribute to the overall hybrid which is how the molecule truly exists in reality. Lets draw the three resonance structures for the nitrate anion no 3.
How To Move Electrons To Transform One Resonance Structure Into
 How Are These Two Organic Resonance Structures Different
How Are These Two Organic Resonance Structures Different
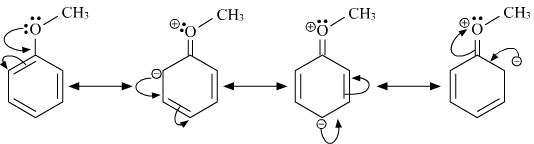 What Are The Resonance Structures Of Anisole And Benzaldehyde
What Are The Resonance Structures Of Anisole And Benzaldehyde
 Why Can One Double Bond Exist In Some Resonance Structures But Two
Why Can One Double Bond Exist In Some Resonance Structures But Two
 6 2 Resonance Organic Chemistry 1 An Open Textbook
6 2 Resonance Organic Chemistry 1 An Open Textbook
 The Predicted Stabilities Of Resonance Contributors Mcc Organic
The Predicted Stabilities Of Resonance Contributors Mcc Organic
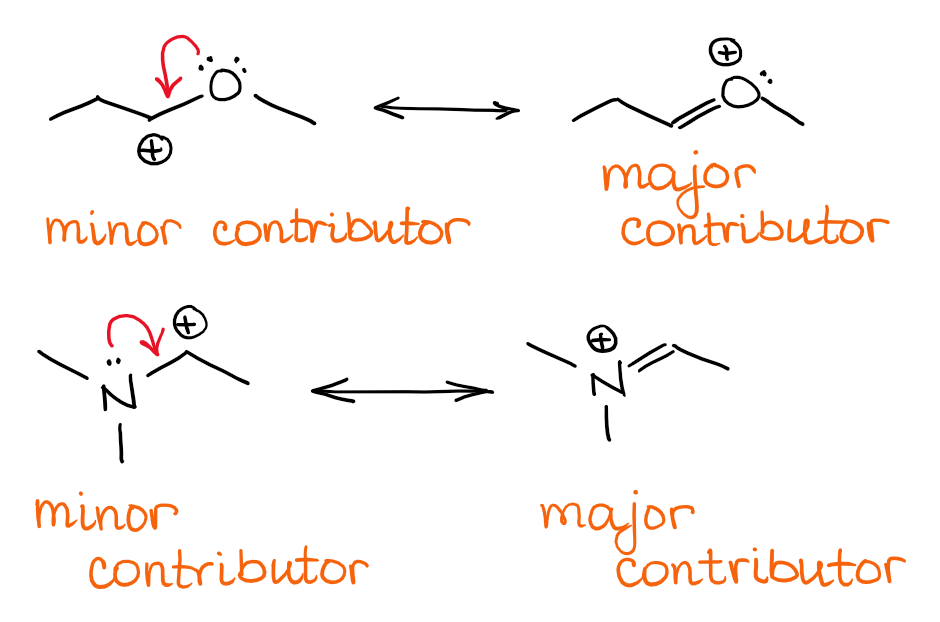 Resonance Structures Archives Organic Chemistry Tutor
Resonance Structures Archives Organic Chemistry Tutor
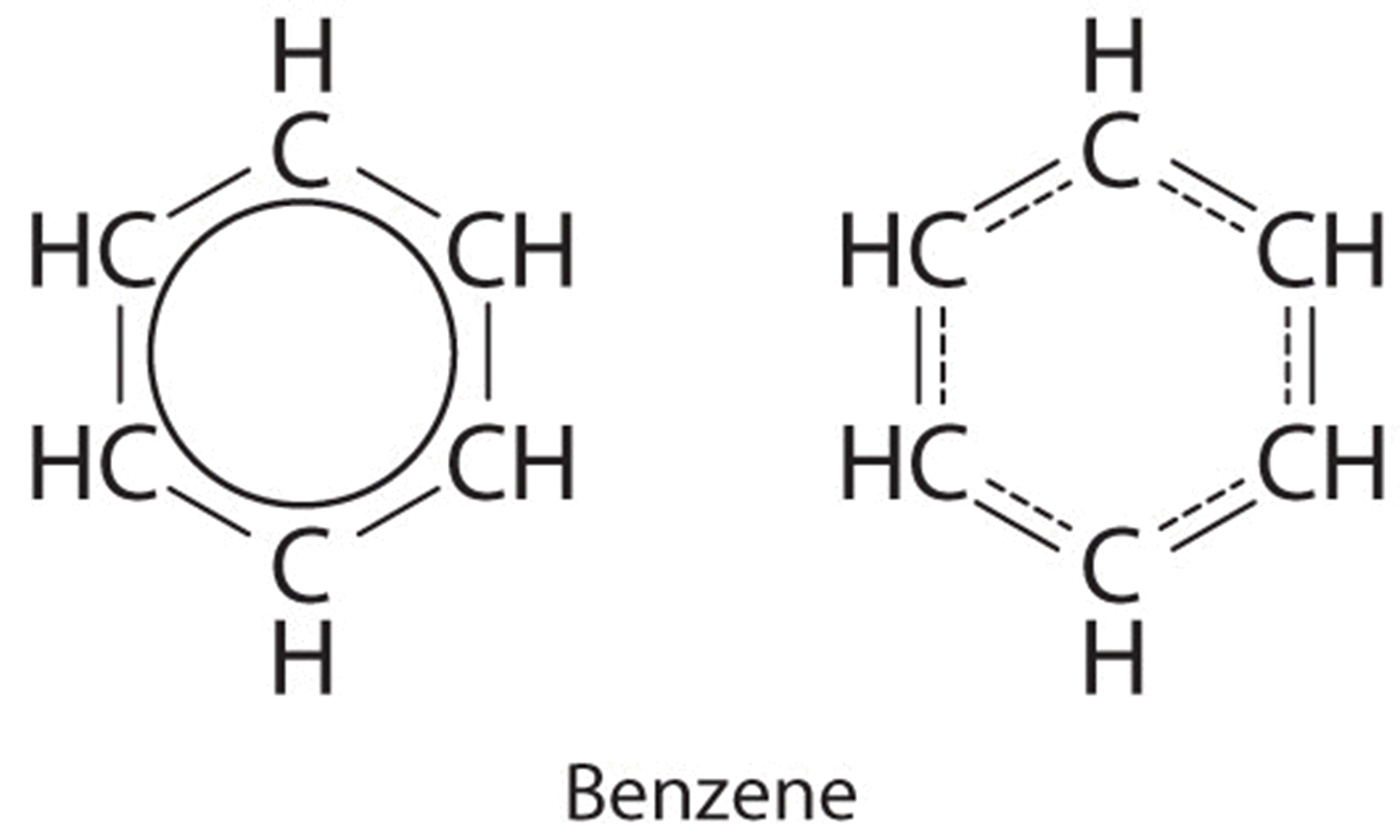 8 6 Resonance Structures Chemistry Libretexts
8 6 Resonance Structures Chemistry Libretexts
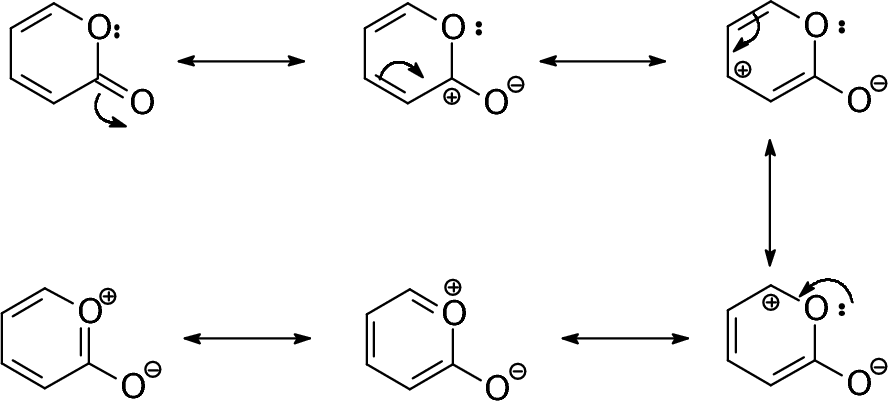
Resonance Structures For Phenol Download Scientific Diagram
How Would You Draw All The Resonance Structures For Nitrate No3
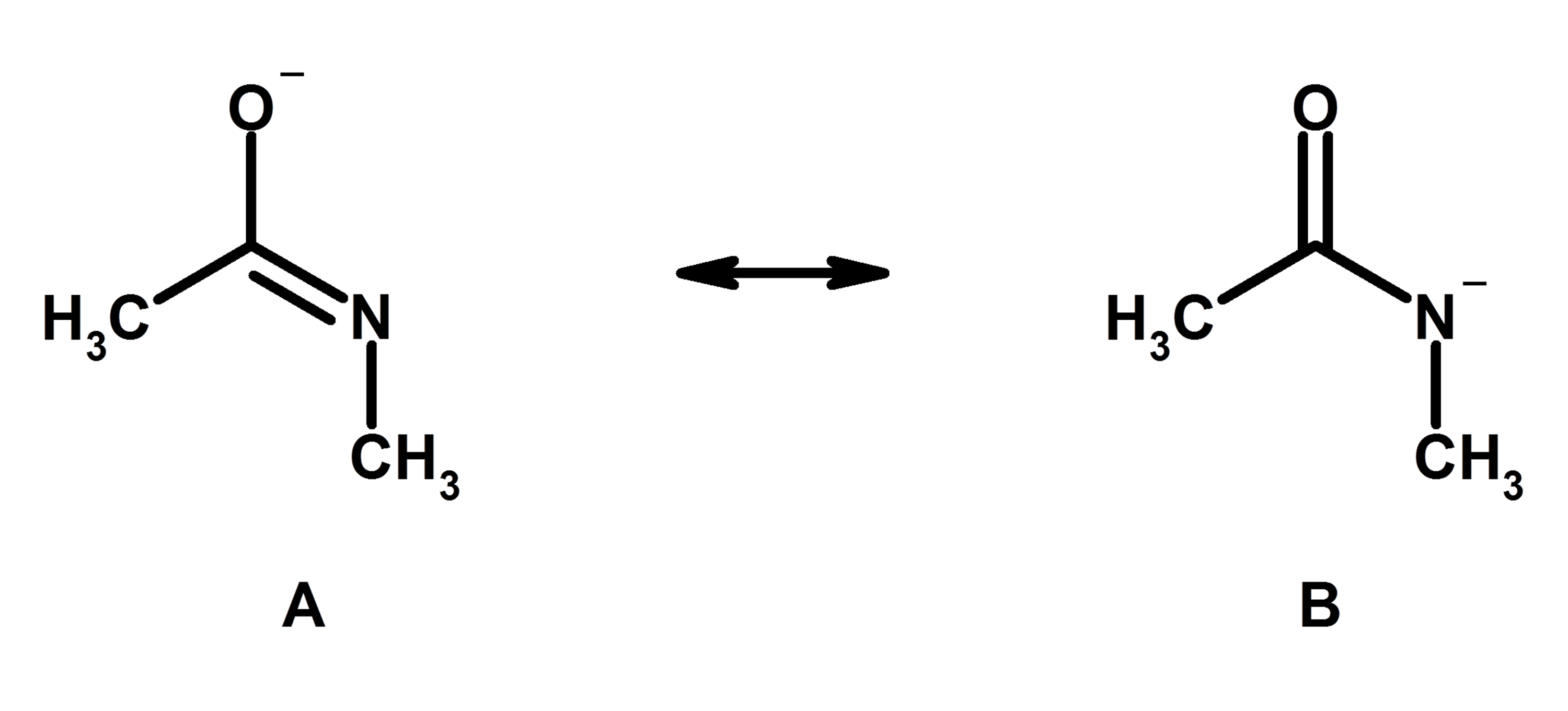 What Is Resonance 7 Rules To Master It Free Guide Organic
What Is Resonance 7 Rules To Master It Free Guide Organic
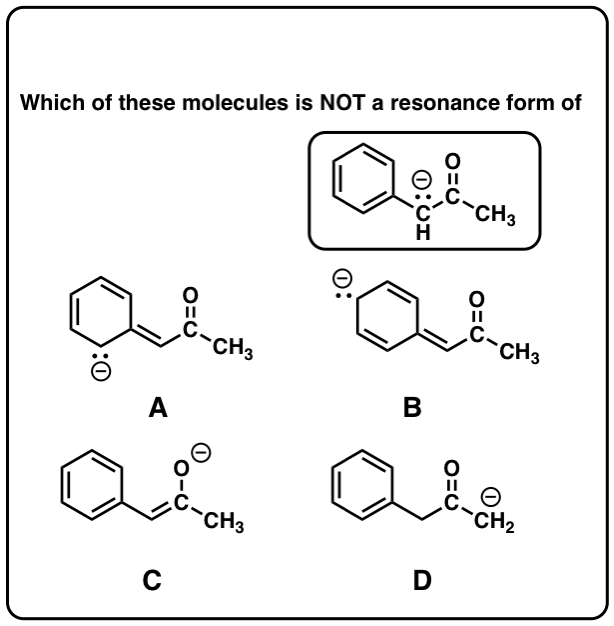 Resonance Structures 4 Rules On How To Evaluate Them With Practice
Resonance Structures 4 Rules On How To Evaluate Them With Practice
 Resonance Chemistry Libretexts
Resonance Chemistry Libretexts
Illustrated Glossary Of Organic Chemistry Resonance Contributor
 Drawing Lewis Structures Resonance Structures Chemistry
Drawing Lewis Structures Resonance Structures Chemistry
 Resonance Structures Resonance Effect Explanation With Examples
Resonance Structures Resonance Effect Explanation With Examples
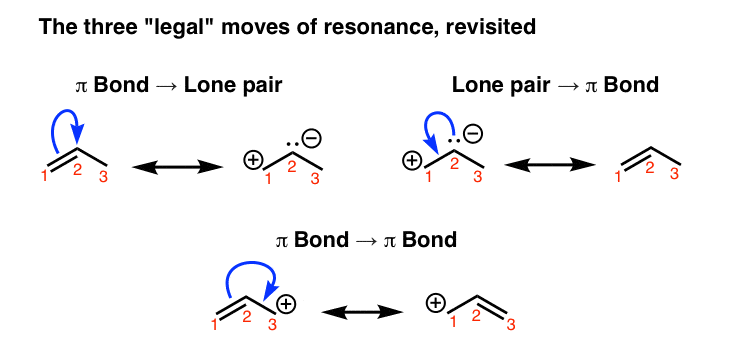 Resonance Structures 4 Rules On How To Evaluate Them With Practice
Resonance Structures 4 Rules On How To Evaluate Them With Practice
How To Assign Major And Minor Resonance Structures
In Chemistry What Are Resonance Structures Quora
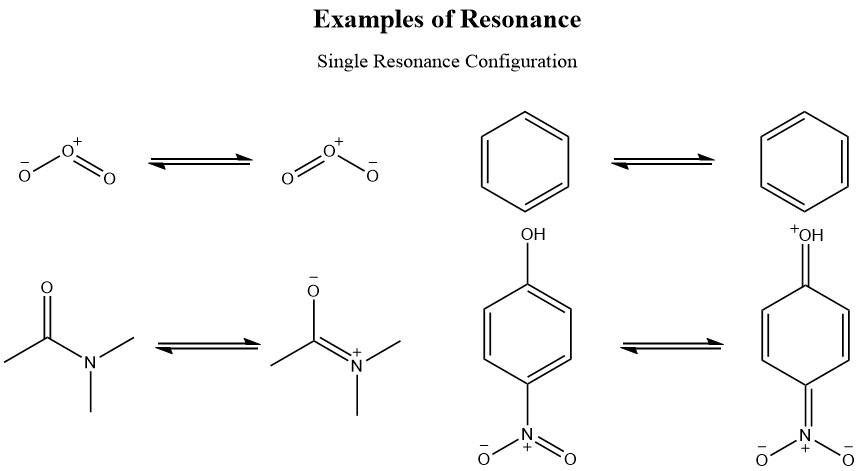 2 5 Rules For Resonance Forms Chemistry Libretexts
2 5 Rules For Resonance Forms Chemistry Libretexts
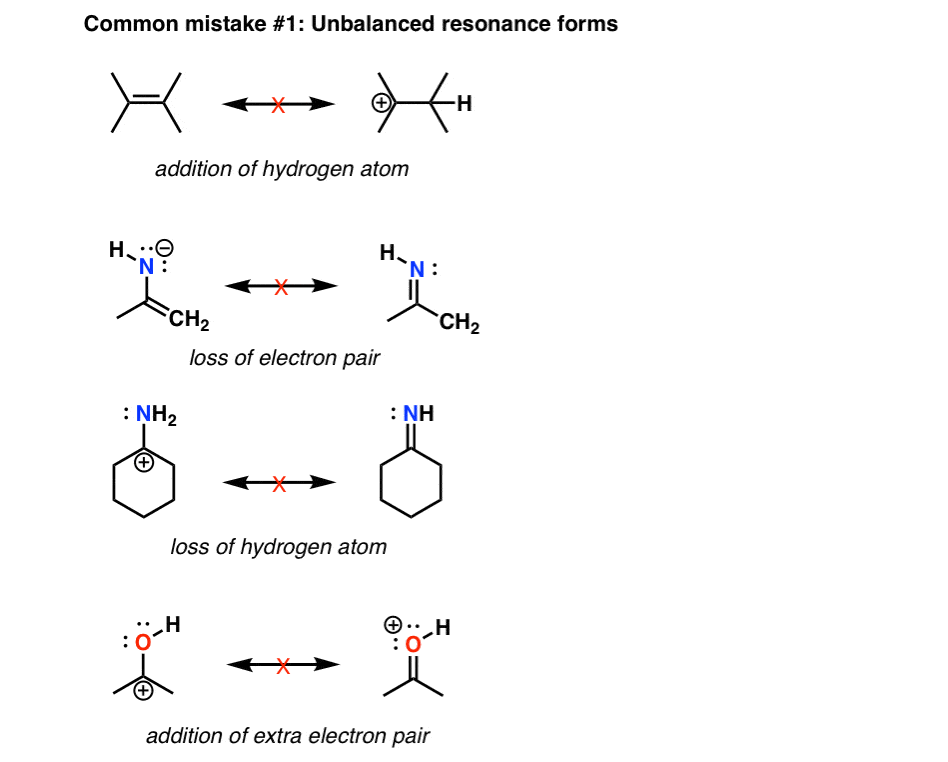 Drawing Resonance Structures 3 Common Mistakes To Avoid
Drawing Resonance Structures 3 Common Mistakes To Avoid
 Resonance Structures The Cavalcade O Chemistry
Resonance Structures The Cavalcade O Chemistry
 5 The Two Lewis Resonance Structures Of The Acetate Anion Viz
5 The Two Lewis Resonance Structures Of The Acetate Anion Viz
How Do Resonance Structures And Isomers Differ Socratic
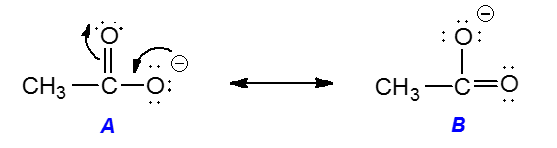 1 5 Resonance Structures Dat Bootcamp
1 5 Resonance Structures Dat Bootcamp
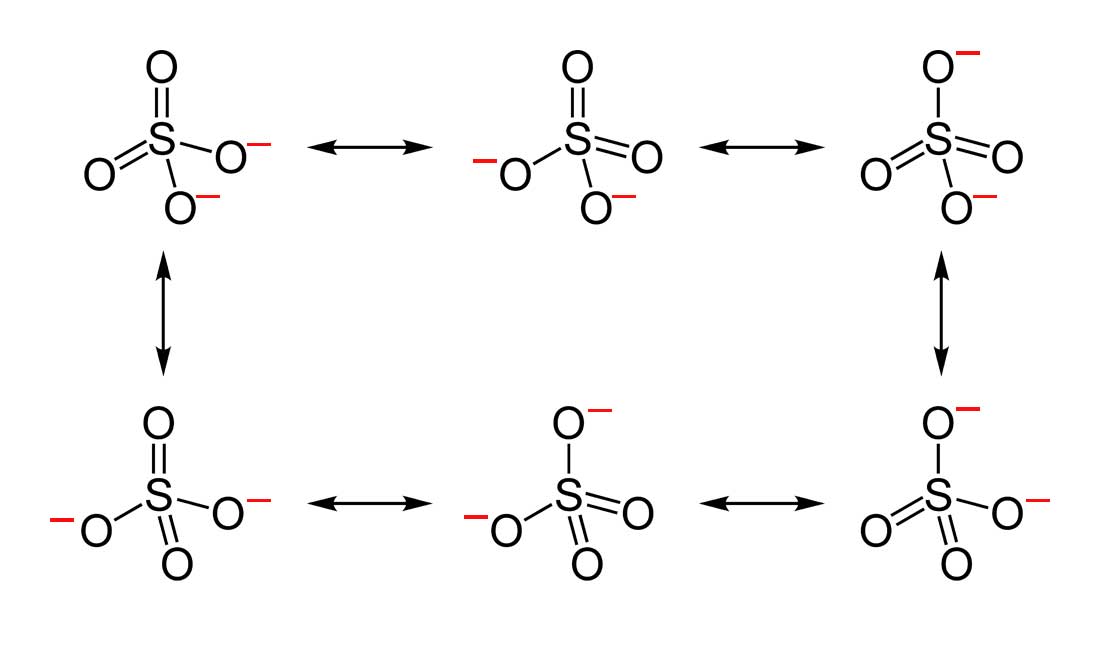
 Which Resonance Structure Of Sulfur Trioxide Represents Its True
Which Resonance Structure Of Sulfur Trioxide Represents Its True
 How To Choose The More Stable Resonance Structure Chemistry Steps
How To Choose The More Stable Resonance Structure Chemistry Steps
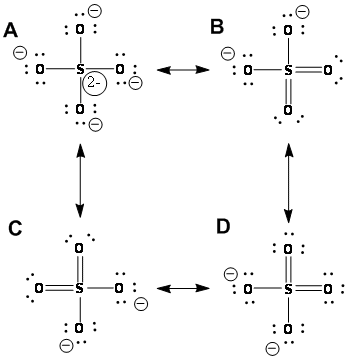 Solved Which Of The Four Resonance Structures For The Sul
Solved Which Of The Four Resonance Structures For The Sul
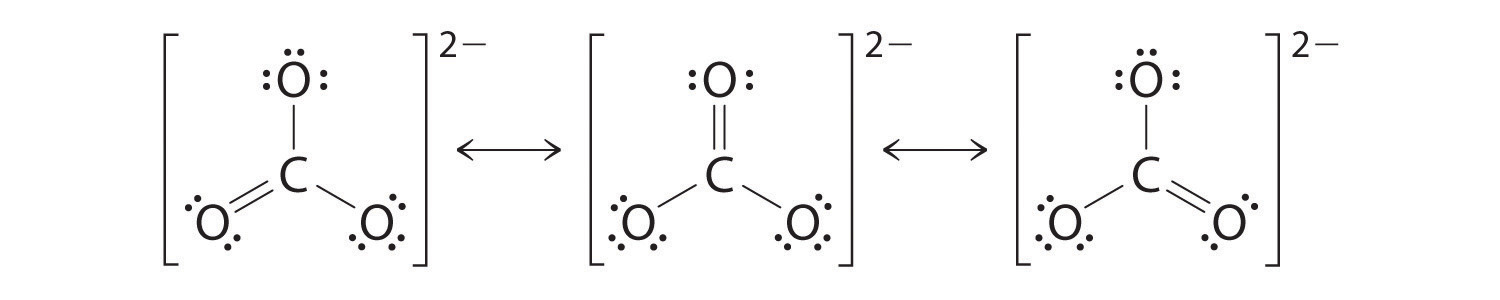 10 5 Resonance Chemistry Libretexts
10 5 Resonance Chemistry Libretexts
 The Predicted Stabilities Of Resonance Contributors Mcc Organic
The Predicted Stabilities Of Resonance Contributors Mcc Organic
How To Write Resonance Structures Quora
 Which Of The Resonance Structures Is More Stable Chemistry
Which Of The Resonance Structures Is More Stable Chemistry
Posting Komentar
Posting Komentar