Graham S Law Of Diffusion Graph
Graham s law states that the rate of diffusion or effusion of a gas is inversely proportional to the square root of its molar mass. This empirical law was stated by scottish chemist thomas graham in 1848.
Graham s law of effusion also called graham s law of diffusion was formulated by scottish physical chemist thomas graham in 1848.

Graham s law of diffusion graph. The equation at the top of this page is graham s law of diffusion named after the scottish chemist thomas graham 1805 1869. R 1 m. Not the usual chemteam writing of graham s law but it still works.
Graham s law states that the effusion rate of a gas is inversely proportional to the square root of the mass of its particles. This formula can be written as. Graham s law of diffusion states that the ratio of the diffusion rate of two gases is the same as the ratio of the square root of the molar mass of the gases.
Other articles where graham s law of diffusion is discussed. Here s a little bit of the making of graham s law. Effusion rates calculate the relative rates of effusion of he g and o 2 g.
Graham measured the rate of. He established the relationship through experiments. This means particles or molecules spread through medium.
Rate 1 rate 2 m 2 m 1. Before we discuss graham s law it is appropriate to know basic definitions of diffusion and effusion. From examining the diffusion of one liquid into another he divided particles into two classes crystalloids such as common salt.
Graham s research on the diffusion of gases was triggered by his reading about the observation of german chemist johann döbereiner that hydrogen gas diffused out of a small crack in a glass bottle faster than the surrounding air diffused in to replace it. It states that the diffusion rate of a gas is inversely proportional to the square root of its molar mass molecular weight. Graham s law of diffusion calculator scroll to the bottom for instructions and four examples.
Effusion refers to the movement of gas particles through a small hole. Graham found experimentally that the rate of effusion of a gas is inversely proportional to the square root of the mass of its particles. R 1 2 r 2 2 mm 2 mm 1.
Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. Graham s law of diffusion or graham s law of effusion is a law that expresses the relationship between the rate of diffusion or effusion to molar masses of particles. Calculate the relative rates of diffusion for h 2 o when compared to d 2 o.
Diffusion is a phenomenon where there is a movement of one material move from area of high concentration to the area of low concentration. See this law in equation form below. He developed graham s law of the diffusion rate of gases and also found that the relative rates of the effusion of gases are comparable to the diffusion rates.
2 the d 2 o is slower so i m going to assign it to r 2 and give it a value of 1. 1 write graham s law.
 Gas Laws Boyle S Law Charle S Law Gay Lussac S Law Avogadro S Law
Gas Laws Boyle S Law Charle S Law Gay Lussac S Law Avogadro S Law
How To Do P V T Pressure Volume Temperature Gas Calculations
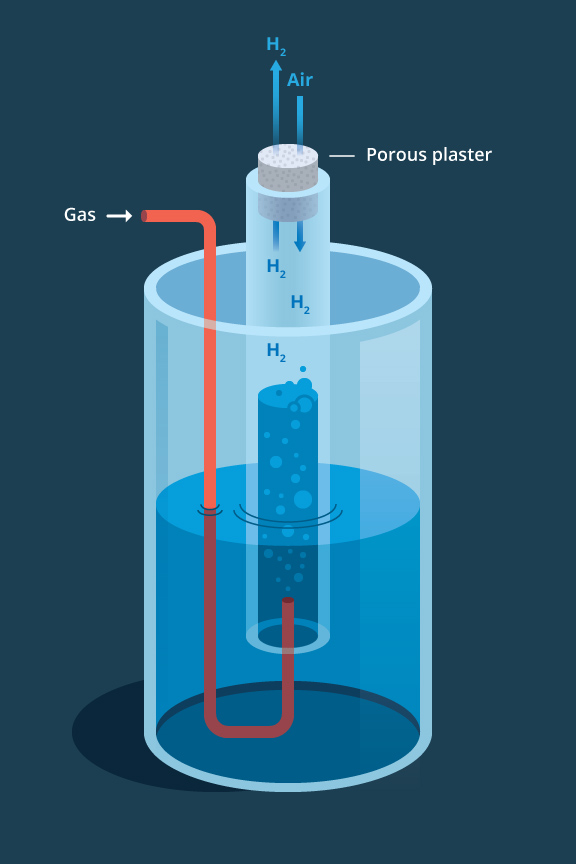 Diffusion I Chemistry Visionlearning
Diffusion I Chemistry Visionlearning
 Video Animation Gas Effusion Graham S Law By Russell Kightley Media
Video Animation Gas Effusion Graham S Law By Russell Kightley Media
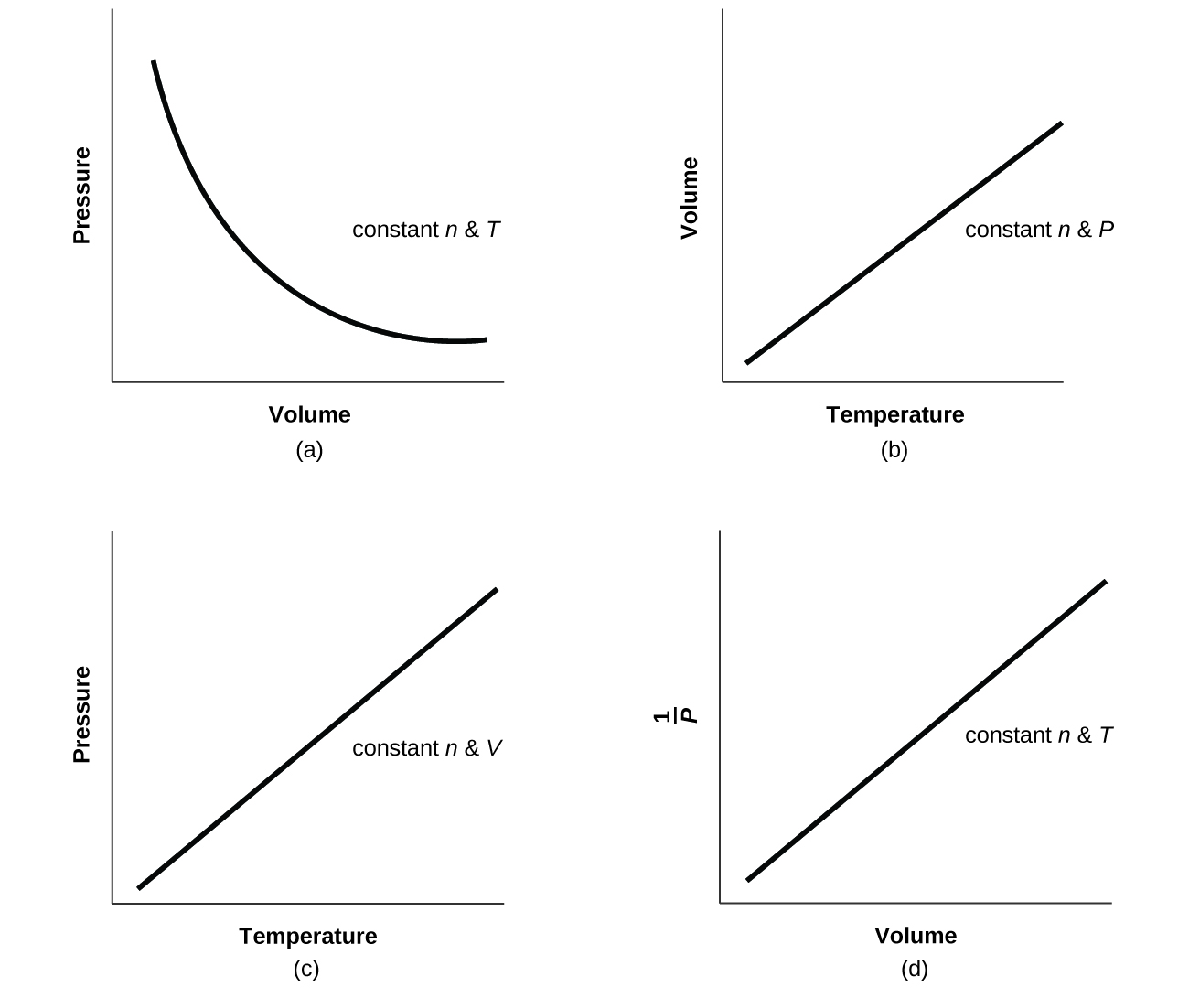 8 E Gases Exercises Chemistry Libretexts
8 E Gases Exercises Chemistry Libretexts
Ch 9 Exercises Chemistry 2e Openstax
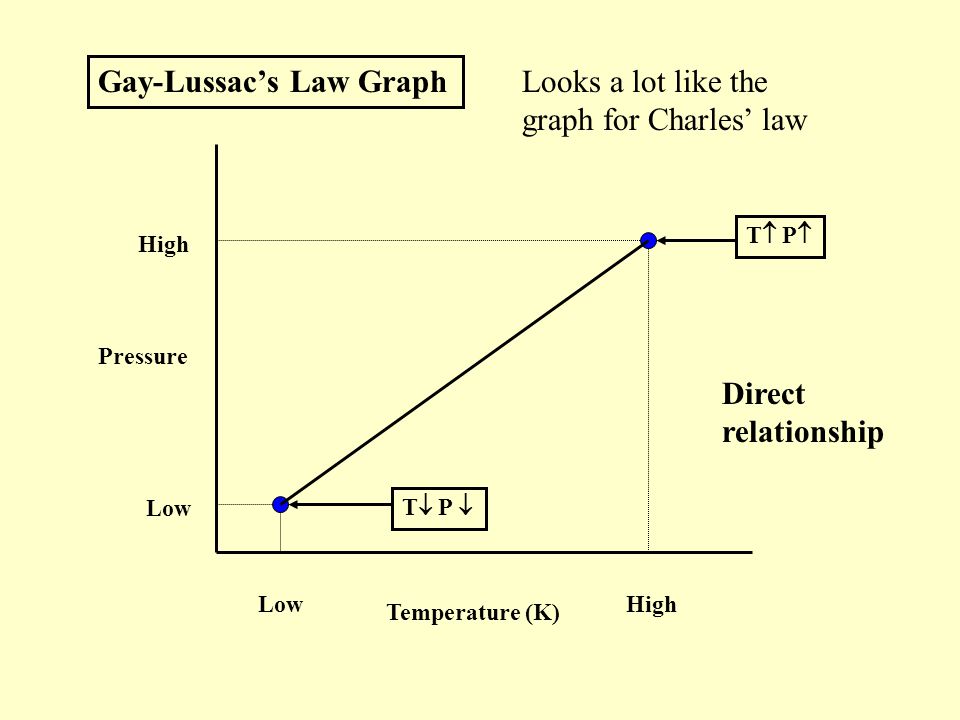 The Gas Laws Pv Nrt Ppt Video Online Download
The Gas Laws Pv Nrt Ppt Video Online Download
 Gas Laws And Properties Of Gases Study Material For Iit Jee
Gas Laws And Properties Of Gases Study Material For Iit Jee
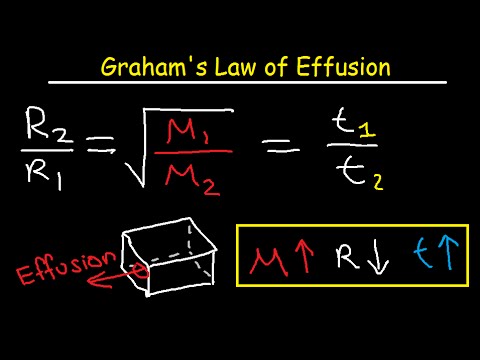 Graham S Law Of Effusion Practice Problems Examples And Formula
Graham S Law Of Effusion Practice Problems Examples And Formula
Phases And Phase Equilibria Mcat Review
 Chem 111a Textbook Notes Spring 2017 Chapter 5 Novella
Chem 111a Textbook Notes Spring 2017 Chapter 5 Novella
Fundamental Gas Laws Relevant To Critical Care Medicine Deranged
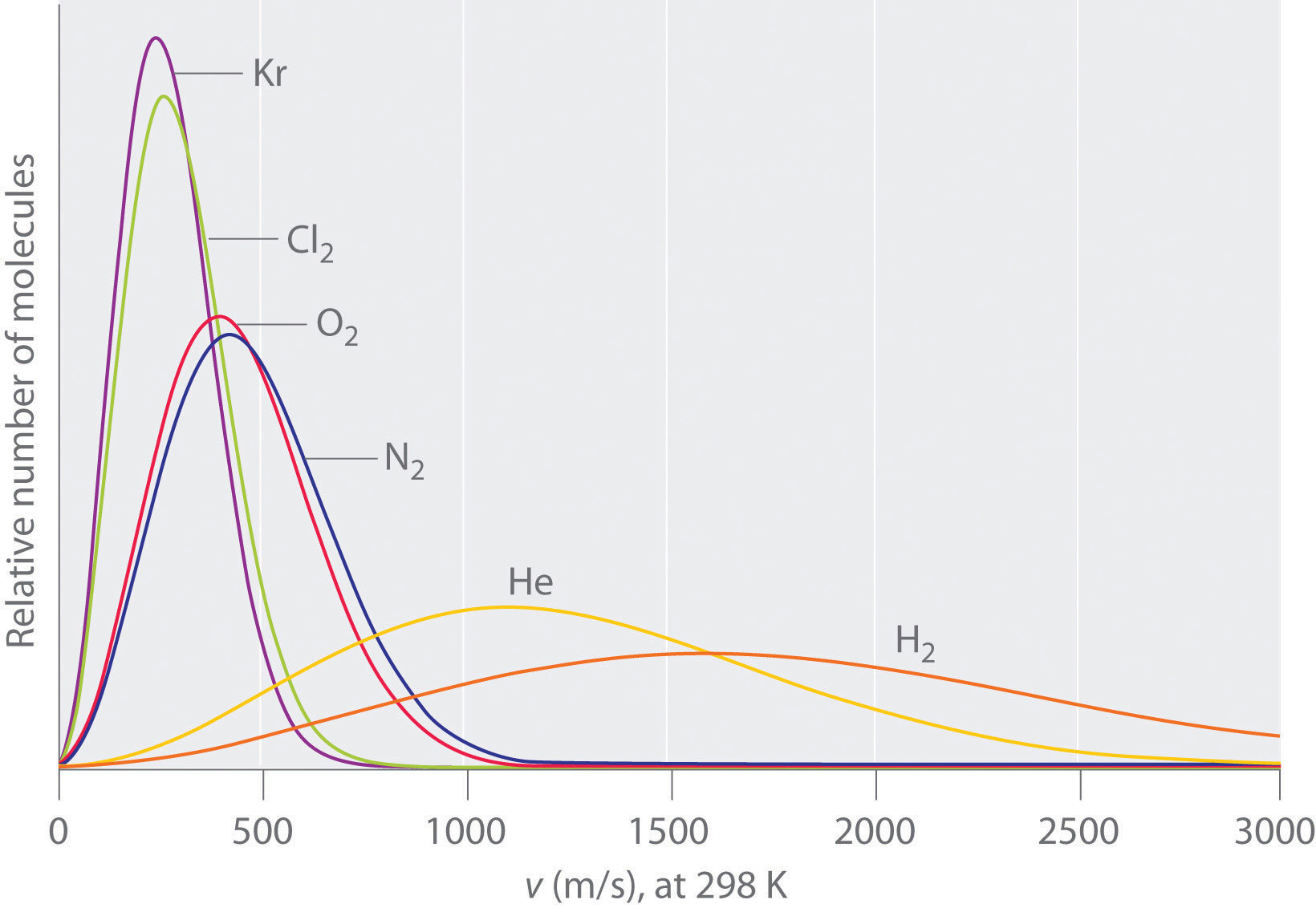 10 6 Diffusion And Effusion Chemistry Libretexts
10 6 Diffusion And Effusion Chemistry Libretexts
Gas Laws Graham S Law Of Effusion
 Graham S Law Of Diffusion And Effusion Chemistrygod
Graham S Law Of Diffusion And Effusion Chemistrygod
Phases And Phase Equilibria Mcat Review
 Graham S Law Of Diffusion And Effusion Chemistrygod
Graham S Law Of Diffusion And Effusion Chemistrygod
 C7 Effusion Diffusion And Grahams Law Hl Ib Chemistry Youtube
C7 Effusion Diffusion And Grahams Law Hl Ib Chemistry Youtube
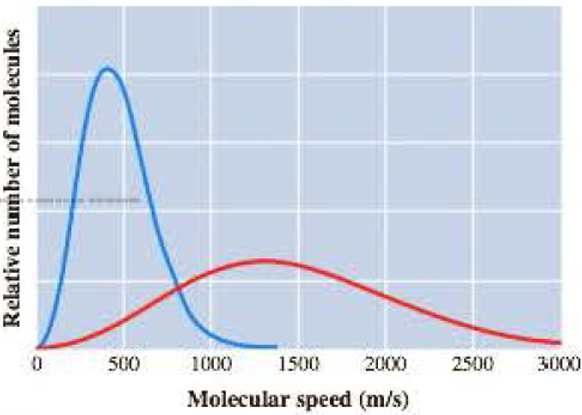 The Graph Here Represents The Distribution Of Molecular Speeds Of
The Graph Here Represents The Distribution Of Molecular Speeds Of
 Co2 Escapes A Balloon Faster Than Helium When Graham S Law
Co2 Escapes A Balloon Faster Than Helium When Graham S Law
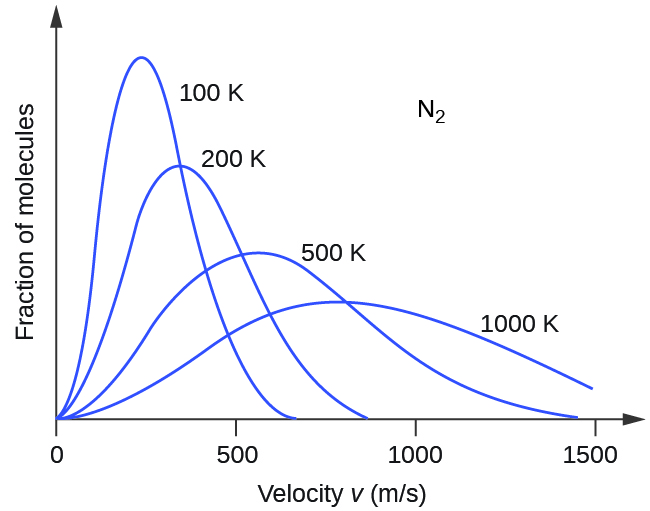 9 5 The Kinetic Molecular Theory Chemistry
9 5 The Kinetic Molecular Theory Chemistry
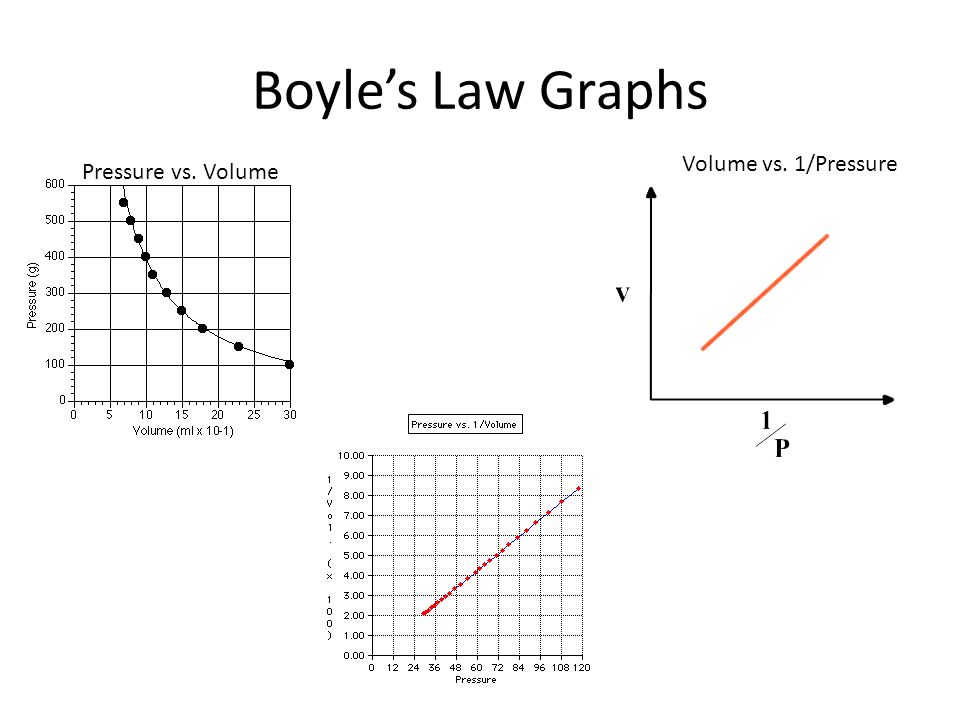 Ideal Gas Law Ppt Video Online Download
Ideal Gas Law Ppt Video Online Download

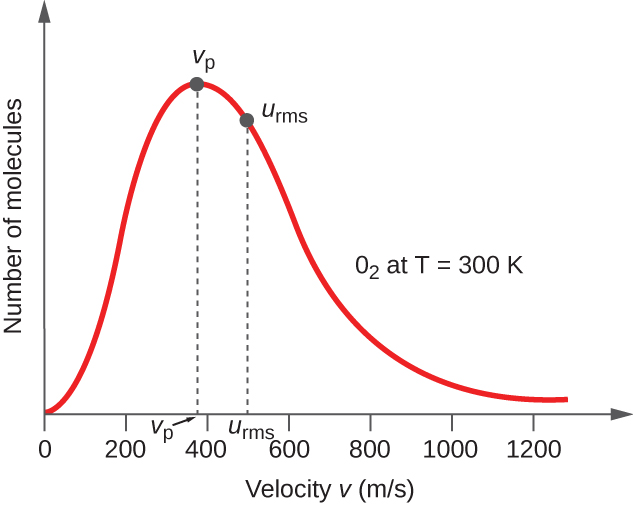 9 5 The Kinetic Molecular Theory Chemistry
9 5 The Kinetic Molecular Theory Chemistry
 Gas Laws Boyle S Law Charle S Law Gay Lussac S Law Avogadro S Law
Gas Laws Boyle S Law Charle S Law Gay Lussac S Law Avogadro S Law
 Diffusion Of Gases Through The Alveolar Membrane Deranged Physiology
Diffusion Of Gases Through The Alveolar Membrane Deranged Physiology
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcszbkxzhxddw60leed Jzlcugtpxolc0pvdcgwznedieyahbwt0 Usqp Cau
 Graham S Law Of Diffusion And Effusion Chemistrygod
Graham S Law Of Diffusion And Effusion Chemistrygod
 Diffusion And Effusion Graham S Law Video Lesson Transcript
Diffusion And Effusion Graham S Law Video Lesson Transcript
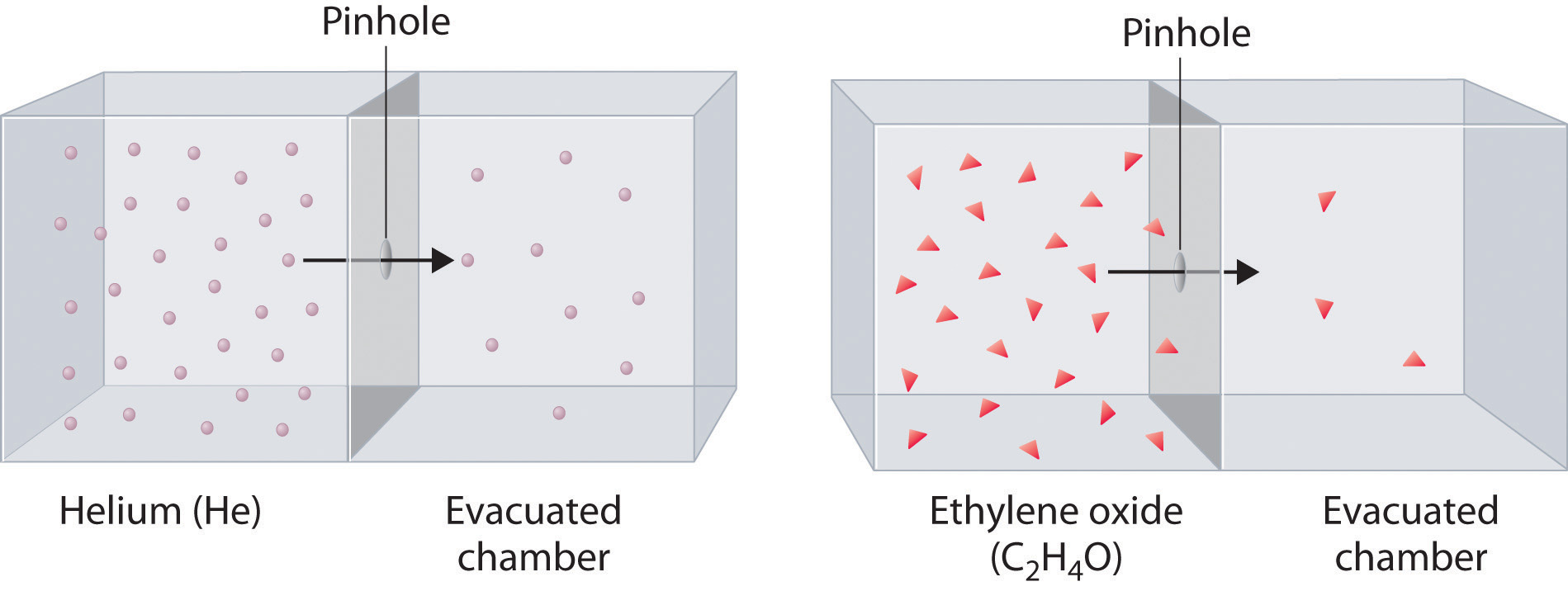 2 9 Graham S Laws Of Diffusion And Effusion Chemistry Libretexts
2 9 Graham S Laws Of Diffusion And Effusion Chemistry Libretexts
How To Do P V T Pressure Volume Temperature Gas Calculations
 Graham S Law Of Diffusion Video Khan Academy
Graham S Law Of Diffusion Video Khan Academy



Posting Komentar
Posting Komentar