Maxwell Distribution Of Speeds
I e any one molecule is highly unlikely to grab much more than its average share of the total energy available to all the molecules. Msd can only be applied when dealing with an ideal gas.
 Maxwell Distribution Vose Software
Maxwell Distribution Vose Software
Unlike this previous post the collision detection and dynamics are handled using numpy arrays without explicit python loops except over collision pairs which improves the performance greatly.
Maxwell distribution of speeds. For this reason this function of the relative frequency density f v is also called maxwell boltzmann distribution function. While it is not necessary for the lab to know the mathematical equation it is important to note a few general features of the maxwell distribution. This script demonstrates the relaxation of an ensemble of colliding particles towards the equilibrium maxwell boltzmann distribution of their speeds.
This predictable distribution of molecular speeds is known as the maxwell boltzmann distribution after its originators who calculated it based on kinetic theory and it has since been confirmed experimentally figure 2 15. The maxwell boltzmann equation which forms the basis of the kinetic theory of gases defines the distribution of speeds for a gas at a certain temperature. Another useful quantity is known as the.
This longer tail pulls the average speed slightly to the right of the peak of the graph. Maxwell speed distribution directly from boltzmann distribution fundamental to our understanding of classical molecular phenomena is the boltzmann distribution which tells us that the probability that any one molecule will be found with energy e decreases exponentially with energy. The entire distribution of speeds is shifted by changes in temperature.
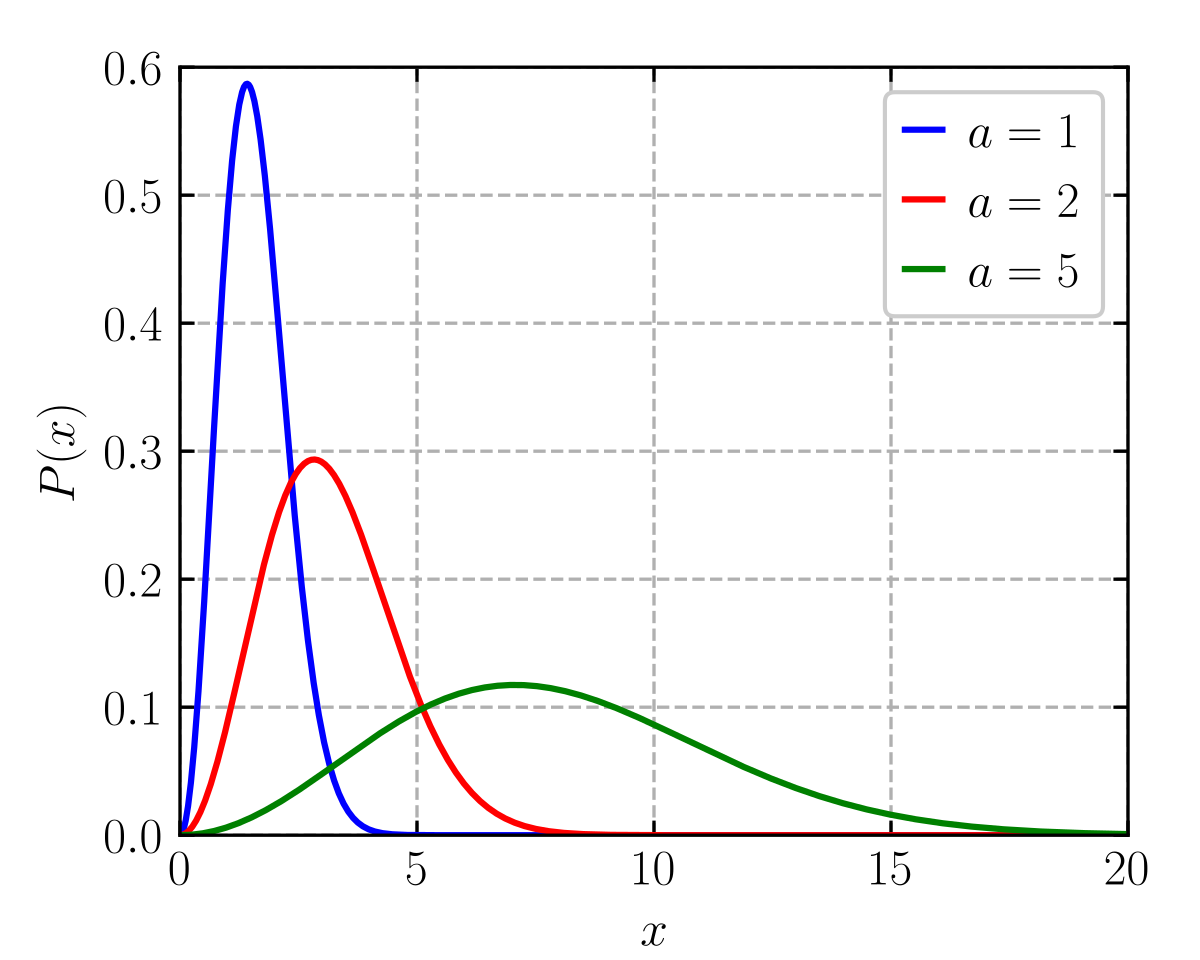
Msd is a probability distribution describing the dispersion of these molecular speeds. Mathematically the maxwell boltzmann distribution is the chi distribution with three degrees of freedom the components of the velocity vector in euclidean space with a scale parameter measuring speeds in units proportional to the square root of displaystyle t m the ratio of temperature and particle mass. Using statistical methods physicists james clerk maxwell and ludwig boltzmann were able to derive the following formula for the molecular speed distribution in an ideal gas.
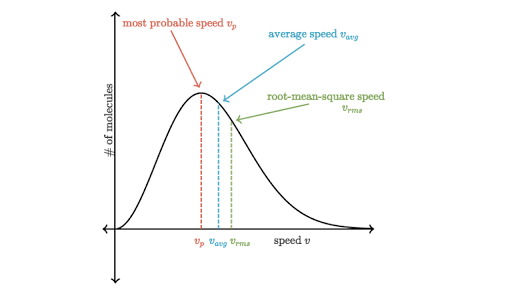
From this distribution function the most probable speed the average speed and the root mean square speed can be derived. The reason the average speed is located to the right of the peak is due to the longer tail on the right side of the maxwell boltzmann distribution graph. This leads us to maxwell s speed distribution msd named after james clerk maxwell to whom this theory is attributed.
The distribution has a crude bell shape which peaks at vmp 2kt m 1 2 the most probable velocity.
2 4 Distribution Of Molecular Speeds University Physics Volume 2
 How To Explain The Maxwell Boltzmann Distribution Graph
How To Explain The Maxwell Boltzmann Distribution Graph
 27 2 The Distribution Of The Components Of Molecular Speeds Are
27 2 The Distribution Of The Components Of Molecular Speeds Are
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcsqsi5ayxhudyoira7wpd Plde9prarvhozzrzkr Q1gmk32qjt Usqp Cau
 Maxwell Boltzmann Distribution Definition Deepai
Maxwell Boltzmann Distribution Definition Deepai
 Maxwell Boltzmann Distribution Of Speed Pt 1 Mp4 Youtube
Maxwell Boltzmann Distribution Of Speed Pt 1 Mp4 Youtube
 Maxwell Boltzmann Distribution Definition Deepai
Maxwell Boltzmann Distribution Definition Deepai
 Maxwellian Velocity Distribution Astrobaki
Maxwellian Velocity Distribution Astrobaki

 Derivation Of Maxwell Boltzmann Distribution Byju S
Derivation Of Maxwell Boltzmann Distribution Byju S
2 4 Distribution Of Molecular Speeds University Physics Volume 2
Kinetic Temperature Thermal Energy
 The Maxwell Boltzmann Distribution
The Maxwell Boltzmann Distribution
 Maxwell Boltzmann Distribution Tec Science
Maxwell Boltzmann Distribution Tec Science
 Kinetic Temperature Thermal Energy
Kinetic Temperature Thermal Energy
Maxwell Boltzmann Distribution

 Why The Maxwell Boltzmann Distribution Curve Is Peaked At U 0
Why The Maxwell Boltzmann Distribution Curve Is Peaked At U 0
 Maxwell Boltzmann Distribution Wikipedia
Maxwell Boltzmann Distribution Wikipedia
 Kinetic Energy And Molecular Speeds Of Gases Definition
Kinetic Energy And Molecular Speeds Of Gases Definition
 Maxwell Boltzmann Distribution
Maxwell Boltzmann Distribution
 Maxwell Boltzmann Distribution Wikipedia
Maxwell Boltzmann Distribution Wikipedia
 Molecular Speed Distributions For Potassium Gas Obtained From The
Molecular Speed Distributions For Potassium Gas Obtained From The
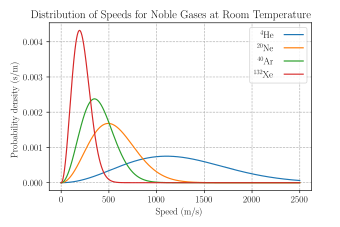
 A Maxwell Boltzmann Distribution For Speed Of Particles In Noble
A Maxwell Boltzmann Distribution For Speed Of Particles In Noble

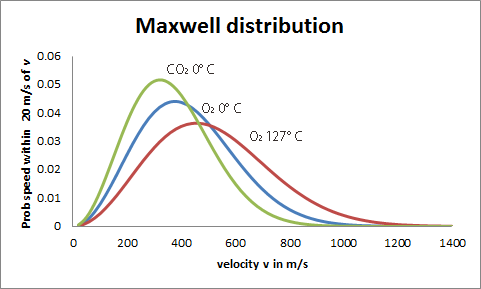 Maxwell S Legacy The Maxwell Distribution
Maxwell S Legacy The Maxwell Distribution
 Maxwell Boltzmann Distribution Wikipedia
Maxwell Boltzmann Distribution Wikipedia
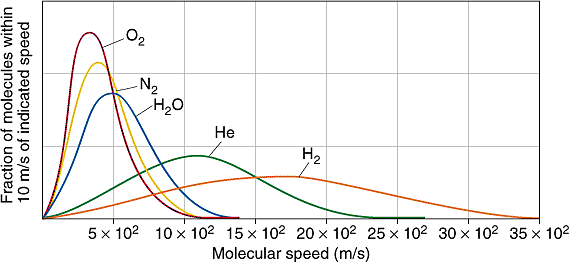 27 3 The Distribution Of Molecular Speeds Is Given By The Maxwell
27 3 The Distribution Of Molecular Speeds Is Given By The Maxwell
 Maxwell Boltzmann Distribution Tec Science
Maxwell Boltzmann Distribution Tec Science
 Maxwell Boltzmann Distributions Chemistry Libretexts
Maxwell Boltzmann Distributions Chemistry Libretexts
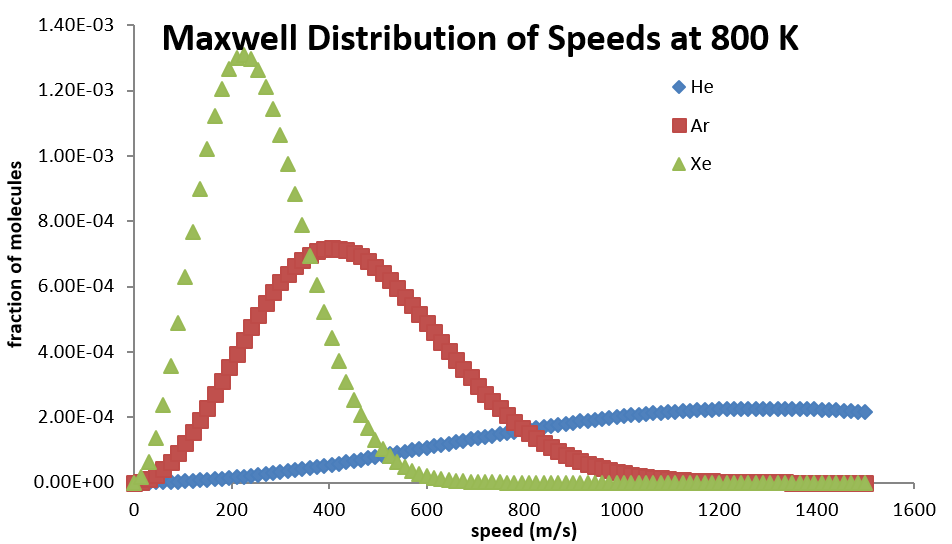 27 2 The Distribution Of The Components Of Molecular Speeds Are
27 2 The Distribution Of The Components Of Molecular Speeds Are
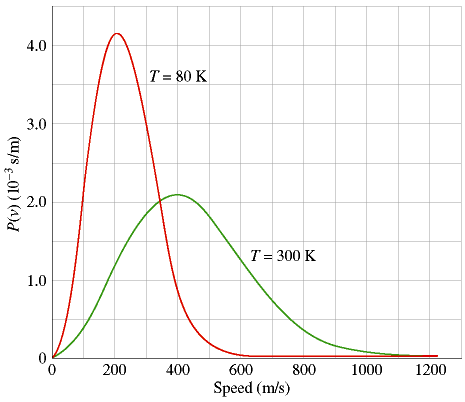 Phyx 103 0 Maxwell Boltzmann Distribution
Phyx 103 0 Maxwell Boltzmann Distribution
Speed Distribution Of Molecules
 The Average Particle Speed In A Maxwell Boltzmann Distribution
The Average Particle Speed In A Maxwell Boltzmann Distribution
Kinetic Temperature Thermal Energy
 Maxwell Boltzmann Distribution Maple Programming Help
Maxwell Boltzmann Distribution Maple Programming Help
The Maxwell Boltzmann Distribution
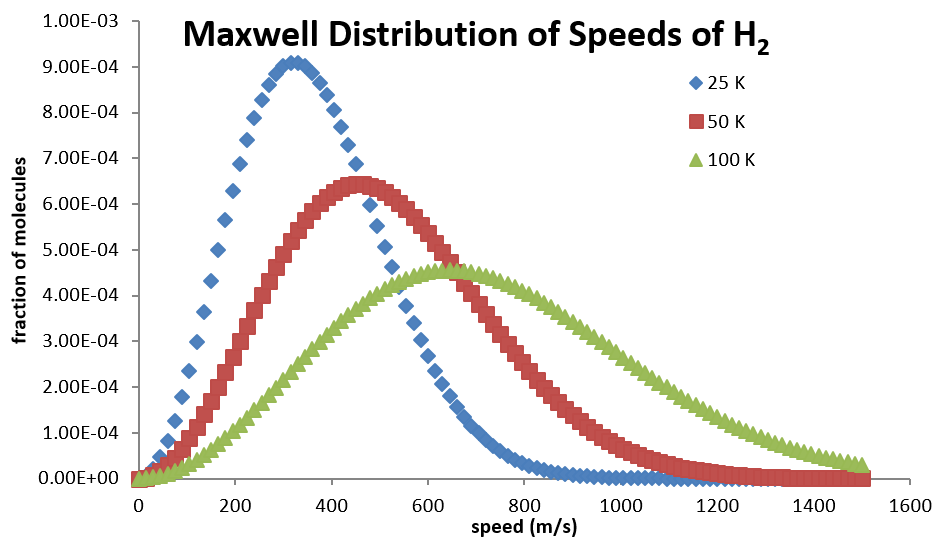
 7 Maxwell Boltzmann Distribution Of Molecular Speeds At Three
7 Maxwell Boltzmann Distribution Of Molecular Speeds At Three
Posting Komentar
Posting Komentar