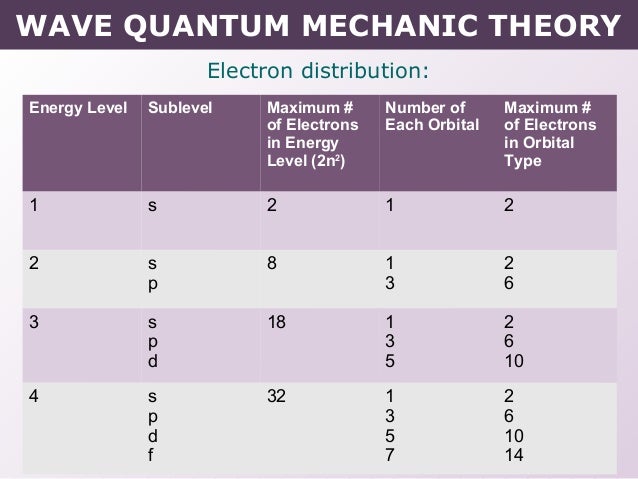
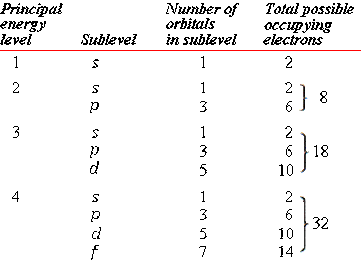
Maximum Number Of Electrons In Each Sublevel
The d sublevel has 5 orbitals so can contain 10 electrons max. Quantum number electronic configuration and shape of orbitals.
 How To Determine The Maximum Number Of Electrons Using Allowed
How To Determine The Maximum Number Of Electrons Using Allowed
The p sublevel has 3 orbitals so can contain 6 electrons max.
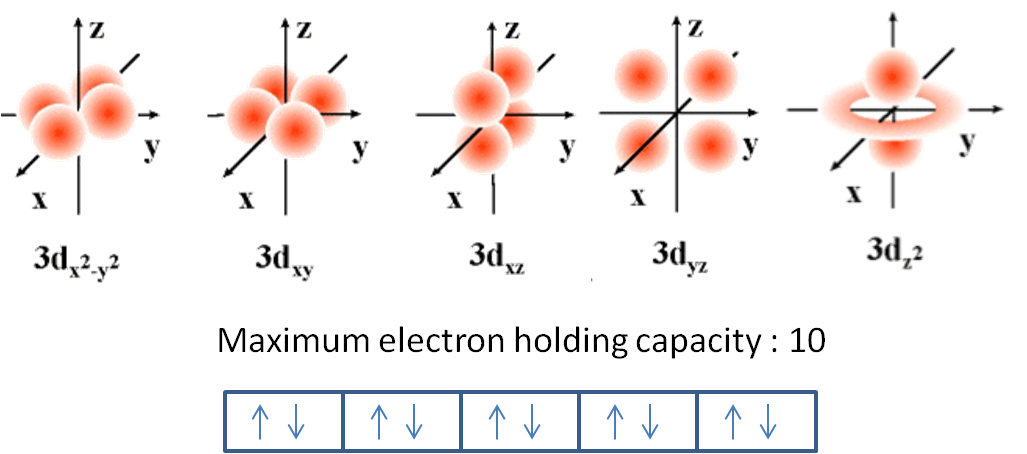
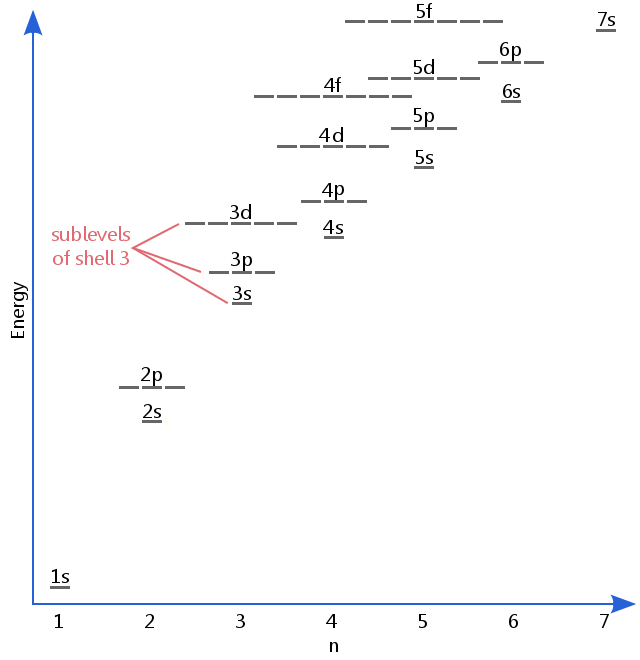
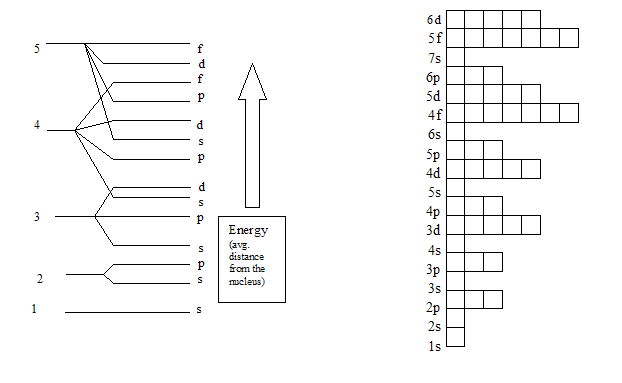
Maximum number of electrons in each sublevel. How many electrons in an atom can have each of the following quantum number or sublevel designations. The 3d 4d etc can each hold ten electrons because they each have five orbitals and each orbital can hold two electrons 5 2 10. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 6 the diagram below really shows the overlap of the principal energy levels on to sublevels and the periodic table.
And the 4 sublevel has 7 orbitals so can contain 14 electrons max. No more than 2 electrons fit in any orbital. The d sublevel has 5 orbitals so can contain 10 electrons max.
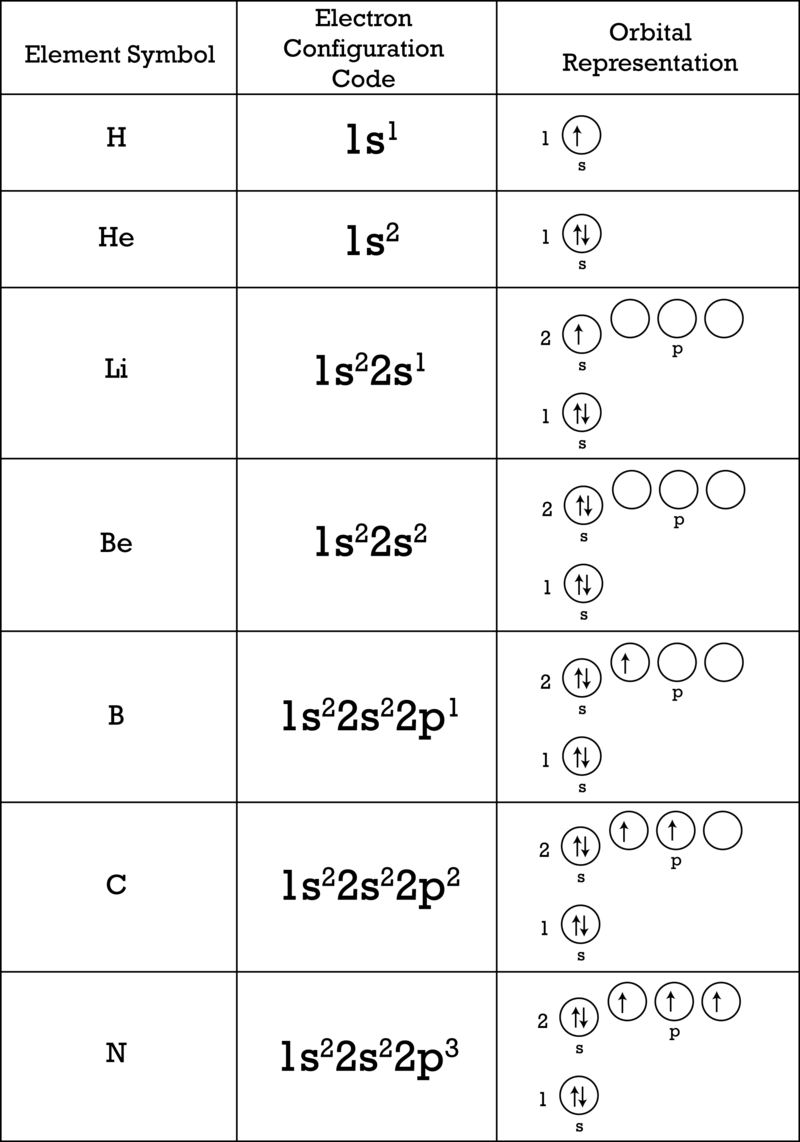
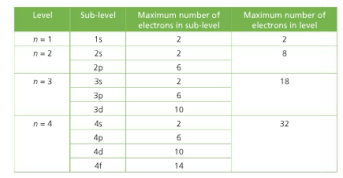
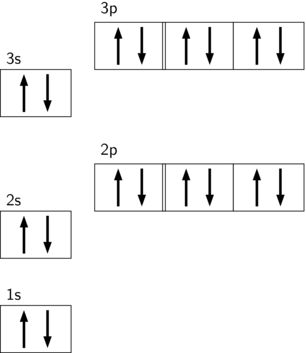
If we have more than one orbital in a sublevel one electron goes in each orbital before you double up hund s. An electron shell is a group of atomic orbitals with a principal quantum number n of the same value. What is the maximum number of electrons that can be contained in the first second third and fourth energy levels respectively.
The s sublevel has just one orbital so can contain 2 electrons max. There can be two electronsin one orbital maximum. The s sublevel has just one orbital so can contain 2 electrons max.
The p sublevel has 3 orbitals so can contain 6 electrons max. N is principal quantum. A n 2 l 1 ml 0 b 5f c n 4 l 1.
Therefore the first shell can hold only 2 electrons the second 8 then 18 25 32 and 50. If we add the number of electrons that each sublevel holds it looks like this. Quantum numbers 1 each orbital in an atom is specified by a set of three quantum numbers n l m and each electron is designated by a set of four quantum numbers n l m and s 2 principle quantum number n i it was proposed by bohr s and denoted by n.
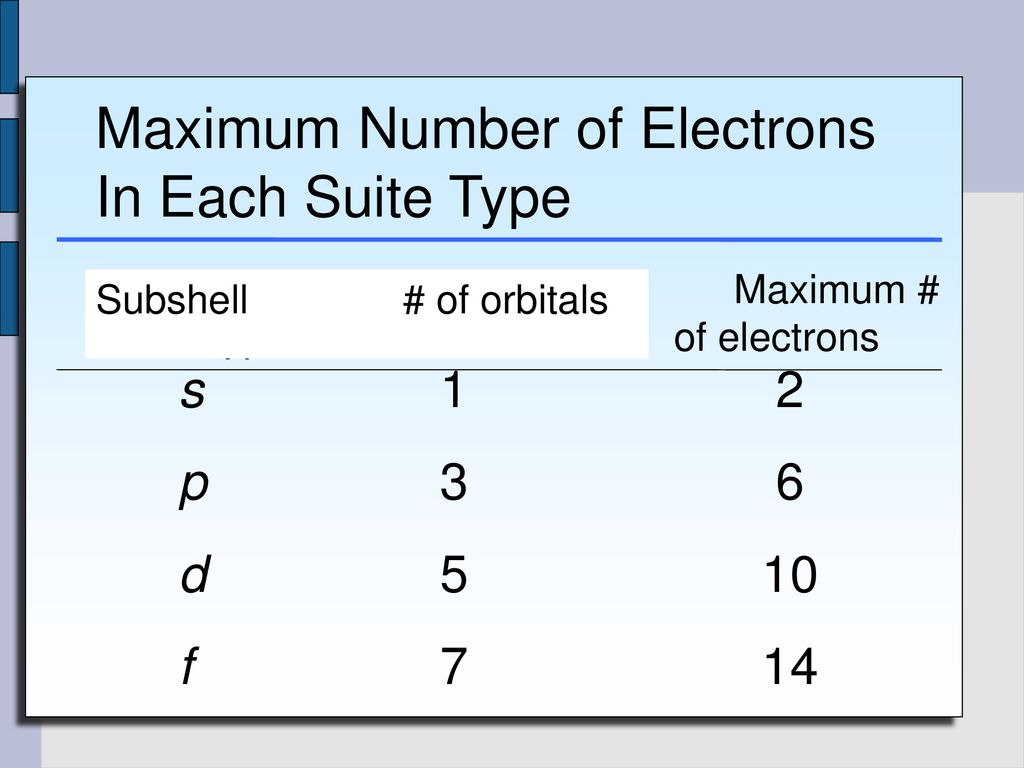
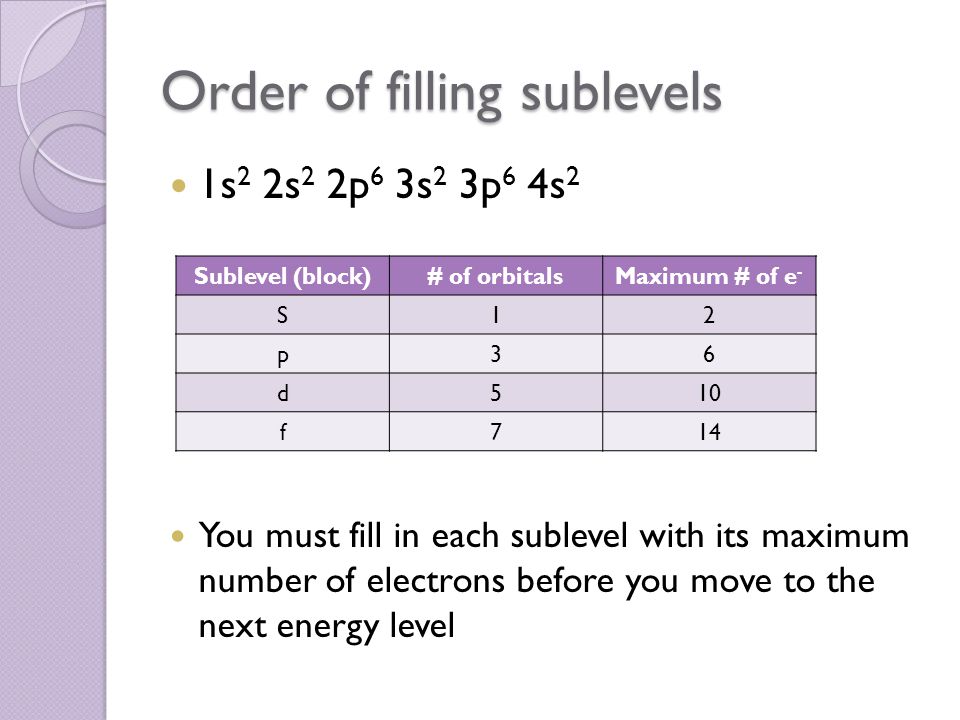
The 2p 3p 4p etc can each hold six electrons because they each have three orbitals that can hold two electrons each 3 2 6. The maximum number of electrons that can be held in each shell can be calculated using the formula 2n 2 where n is the shell number. Each s subshell holds at most 2 electrons each p subshell holds at most 6 electrons each d subshell holds at most 10 electrons.
1st 2 2nd 8 3rd 18 4th 32. Electrons fill the lowest energy levels first. Enter the maximum number of electrons in each type of sublevel s p d and f respectively s 2 p 6 d 10 f 14.
Quantum numbers and shapes of orbitals. Each subshell is constrained to hold 4ℓ 2 electrons at most namely. Thus to find the number of electrons possible per shell.
Electron Configuration Chemistry 10
 Definition Of Sublevel Chemistry Dictionary
Definition Of Sublevel Chemistry Dictionary
 Solved The Maximum Number Of Electrons In Each Sublevel Can No
Solved The Maximum Number Of Electrons In Each Sublevel Can No
 Today S Lesson Electron Hotels Ppt Download
Today S Lesson Electron Hotels Ppt Download
 Summary Of Electron Configurations
Summary Of Electron Configurations
 Electronic Structure Of Atoms Electron Configurations
Electronic Structure Of Atoms Electron Configurations
 Tang 02 Wave Quantum Mechanic Model
Tang 02 Wave Quantum Mechanic Model
What Is The Number Of Orbitals Of A 4d Subshell Quora
 Solved The Maximum Number Of Electrons In Each Sublevel Can No
Solved The Maximum Number Of Electrons In Each Sublevel Can No
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctth F2p Vfrkuaeuotclgi Payigvk1ghhnjeygnpezw3rpbky Usqp Cau
Http Images Pcmac Org Sisfiles Schools Al Trussvillecity Hewitttrussvillemiddle Uploads Documentscategories Documents Atomic Forces Electron Configuration Pdf
 What Is The Maximum Number Of Electrons Which Can Be Accomodated
What Is The Maximum Number Of Electrons Which Can Be Accomodated
Https Web Gccaz Edu Kimld88531 Rev130 Files Wkstatomic Pdf
 The Maximum Number Of Electrons Allowed In An Individual D Orbital
The Maximum Number Of Electrons Allowed In An Individual D Orbital
 Electron Configurations And Orbital Diagrams Maximum Number Of
Electron Configurations And Orbital Diagrams Maximum Number Of
 Solved 8 What Is The Maximum Number Of Electrons That Ca
Solved 8 What Is The Maximum Number Of Electrons That Ca
 8 The Main Energy Level Or Shell Is Given An Integer Number N
8 The Main Energy Level Or Shell Is Given An Integer Number N
 Quantum Numbers Atomic Orbitals And Electron Configurations
Quantum Numbers Atomic Orbitals And Electron Configurations
 Sci C Un5elctrncnfgrtnorbtldgrms
Sci C Un5elctrncnfgrtnorbtldgrms
 Chemistry Chapter 5 Review Flashcards Quizlet
Chemistry Chapter 5 Review Flashcards Quizlet
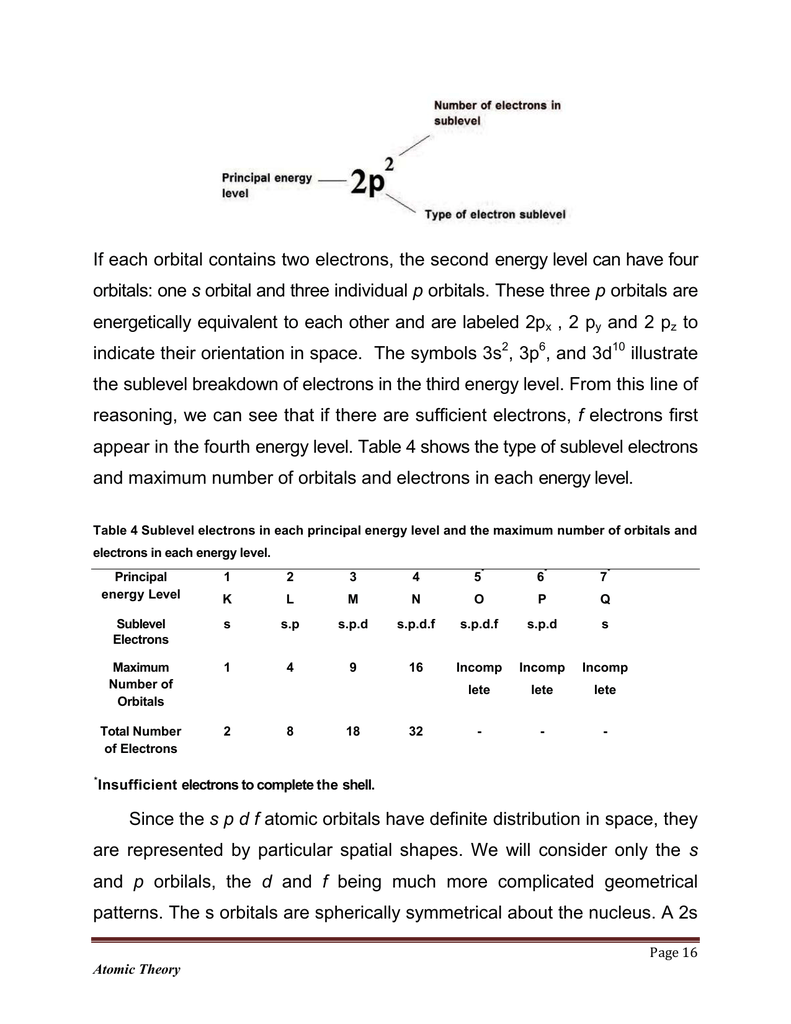 If Each Orbital Contains Two Electrons The Second Energy Level Can
If Each Orbital Contains Two Electrons The Second Energy Level Can
Quantum Numbers And Electronic Structure
 Orbitals And Energy Levels The Atomic Project
Orbitals And Energy Levels The Atomic Project
 What Is The Maximum Number Of Orbitals In The P Sub Level Socratic
What Is The Maximum Number Of Orbitals In The P Sub Level Socratic
 Electrons In Atoms Electron Configuration Orbital Notation Ppt
Electrons In Atoms Electron Configuration Orbital Notation Ppt
 Electron Configuration Atomic Orbitals And Quantum Numbers
Electron Configuration Atomic Orbitals And Quantum Numbers
Https Rankin Instructure Com Courses 393710 Files 33497947 Download Wrap 1
 Solved Reviewi Constants Periodic Table Electrons Are Loc
Solved Reviewi Constants Periodic Table Electrons Are Loc
 Oneclass 33 List The Four Different Sublevels Given That Only A
Oneclass 33 List The Four Different Sublevels Given That Only A
 Electron Configuration Ppt Download
Electron Configuration Ppt Download
 List The Four Different Sublevels And Giv Clutch Prep
List The Four Different Sublevels And Giv Clutch Prep
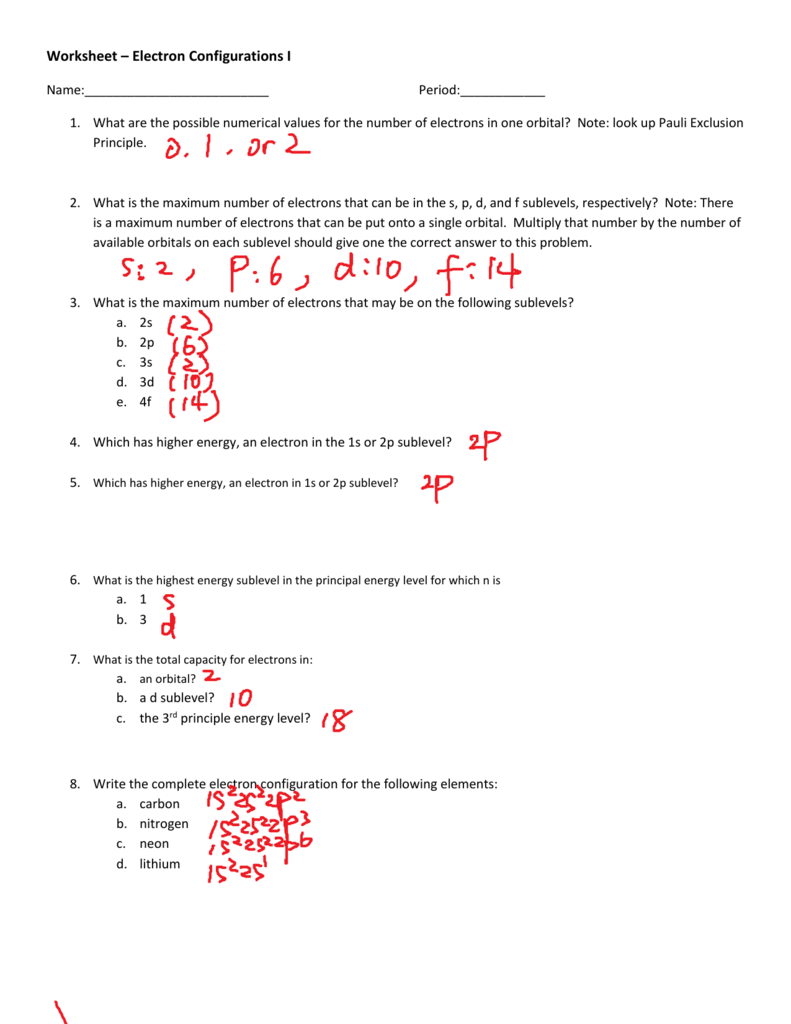 Electron Configurations Worksheet I Answers
Electron Configurations Worksheet I Answers
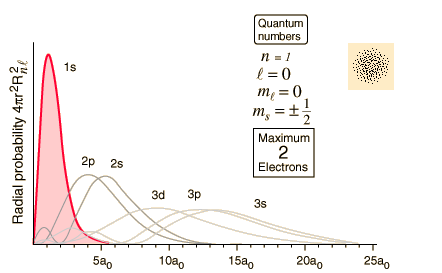
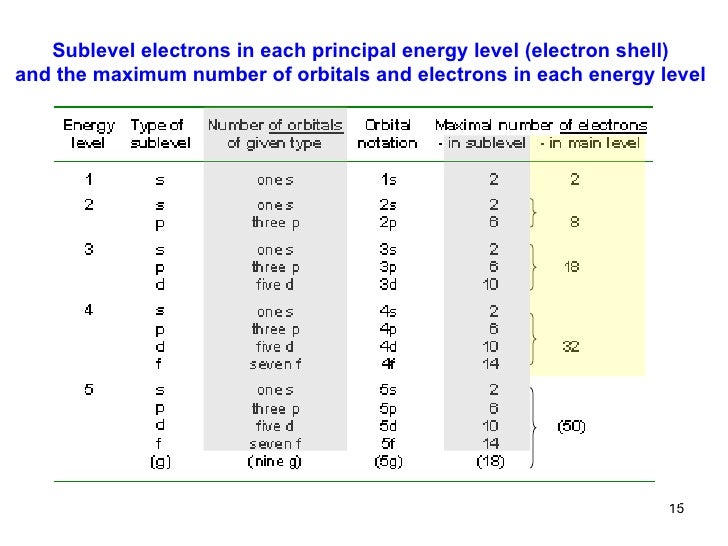
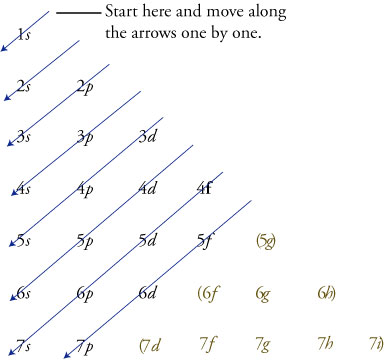
Posting Komentar
Posting Komentar