Open Vs Closed System Chemistry
There are three types of thermodynamics systems. If it does then it is an open system.
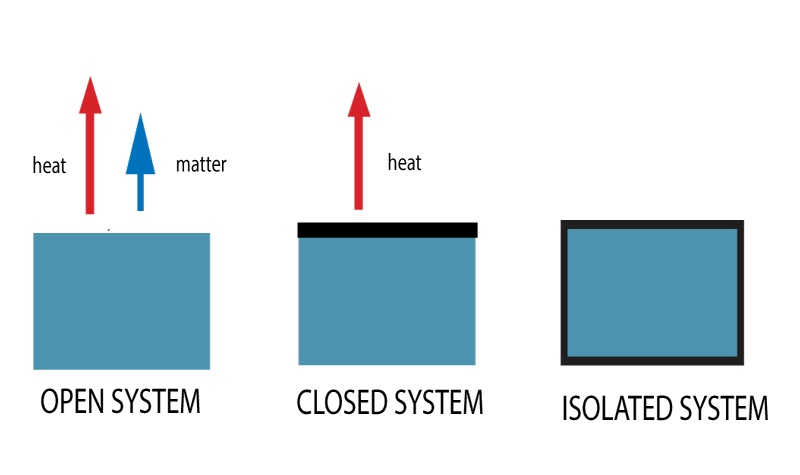 Themodynamic Systems Open Closed Isolated Systems
Themodynamic Systems Open Closed Isolated Systems
A closed system is one where a quantity or series of quantities cannot enter or leave the system.
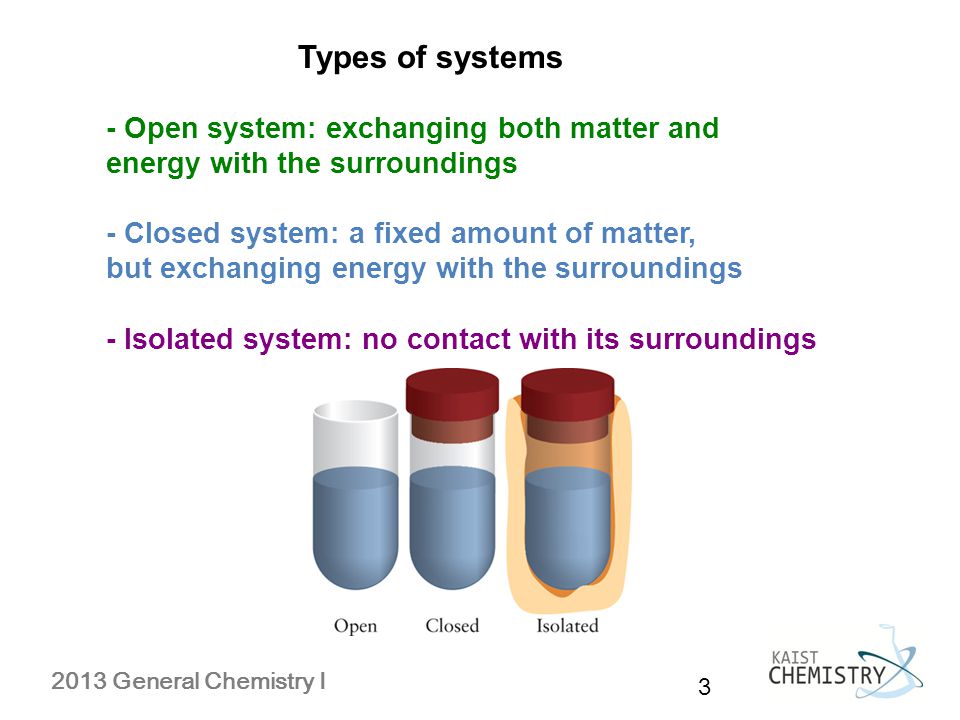
Open vs closed system chemistry. Based on the possible heat and matter transfer they are classified as open closed or isolated systems. A closed system is a system in which matter cannot escape or be added to an environment due to some type of boundary. Closed system definition a closed system is a type of thermodynamic system where mass is conserved within the boundaries of the system but energy is allowed to freely enter or exit the system.
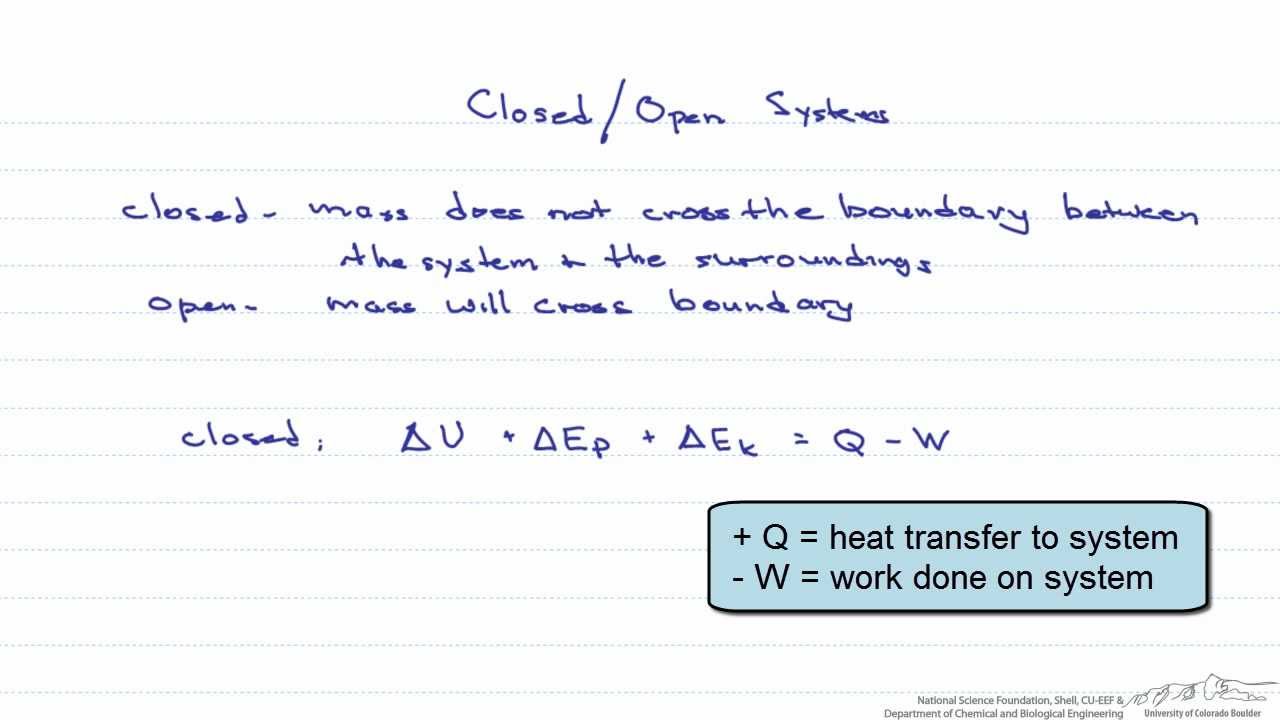
This means that it can both absorb energy and have energy escape from its boundaries. The key difference between closed system and open system is that in a closed system the matter does not exchange with the surrounding but the energy exchanges with the surrounding whereas in an open system both matter and energy exchanges with the surrounding. For example a system might be.
Systems can be either open or closed. System and the surrounding. If it does not then it is either a closed system or an isolated system.
We define a system as something which we identify or prepare in order to do experiments and make observations. In chemistry a closed system is one in which neither reactants nor products can enter or escape yet which allows energy transfer heat and light. Thermodynamics involve the study of heat energy exchange between a system and its surroundings.
Only energy can exit or enter a closed system. A closed system in chemistry refers to a type of a thermodynamic system in which mass is conserved inside the system but energy enters and leaves the system freely. When deciding whether or not something is an open system one must determine whether or not the system allows matter and energy transfer.
Although a closed system is more controlled than an open system however it is still susceptible to outside heat and energy. The system is subject to surrounding factors such as air temperature and pressure. An open system is one that allows energy and matter exchange.
For the purpose of chemistry we can divide the universe into two parts. Open closed and isolated systems in physical chemistry definitions are the key especially at the beginning of learning a new subject. When both energy and matter can be exchanged between the system and its surroundings the system is known as an open system.
Https Www Studeersnel Nl Nl Document Technische Universiteit Delft Chemistry 2 Samenvattingen Summary Chemistry First 2 Weeks 46194 View
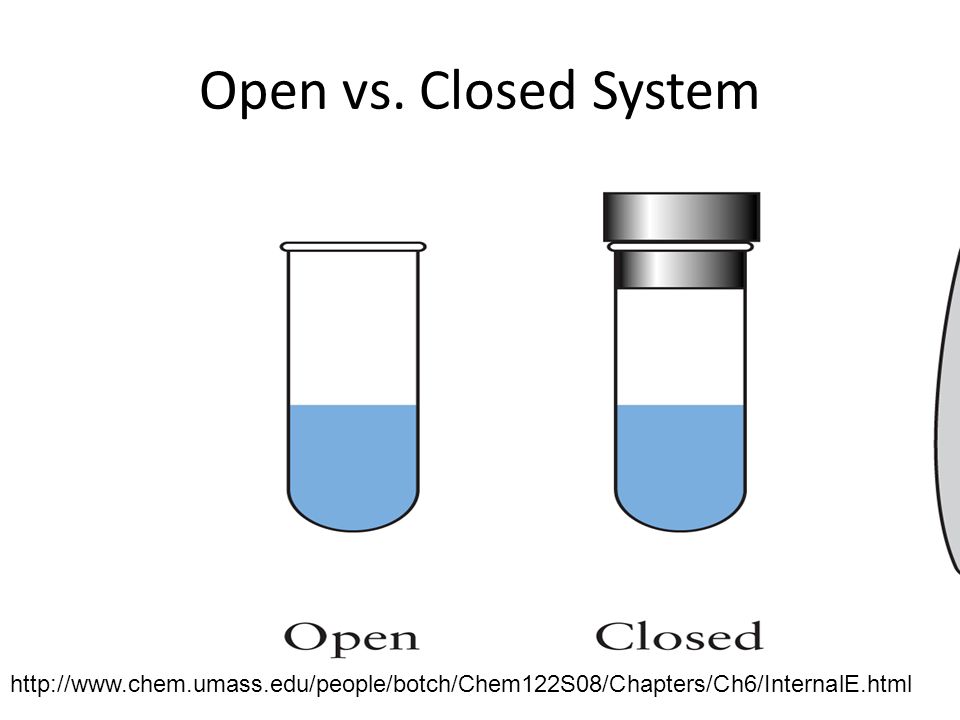
 Open And Closed Systems Youtube
Open And Closed Systems Youtube
Http Www Uvm Edu Cmehrten Courses Earthhist Earth 20closed 20system Pdf
 Open System Systems Theory Wikipedia
Open System Systems Theory Wikipedia
 Chapter 7 Thermodynamics The First Law Ppt Video Online Download
Chapter 7 Thermodynamics The First Law Ppt Video Online Download
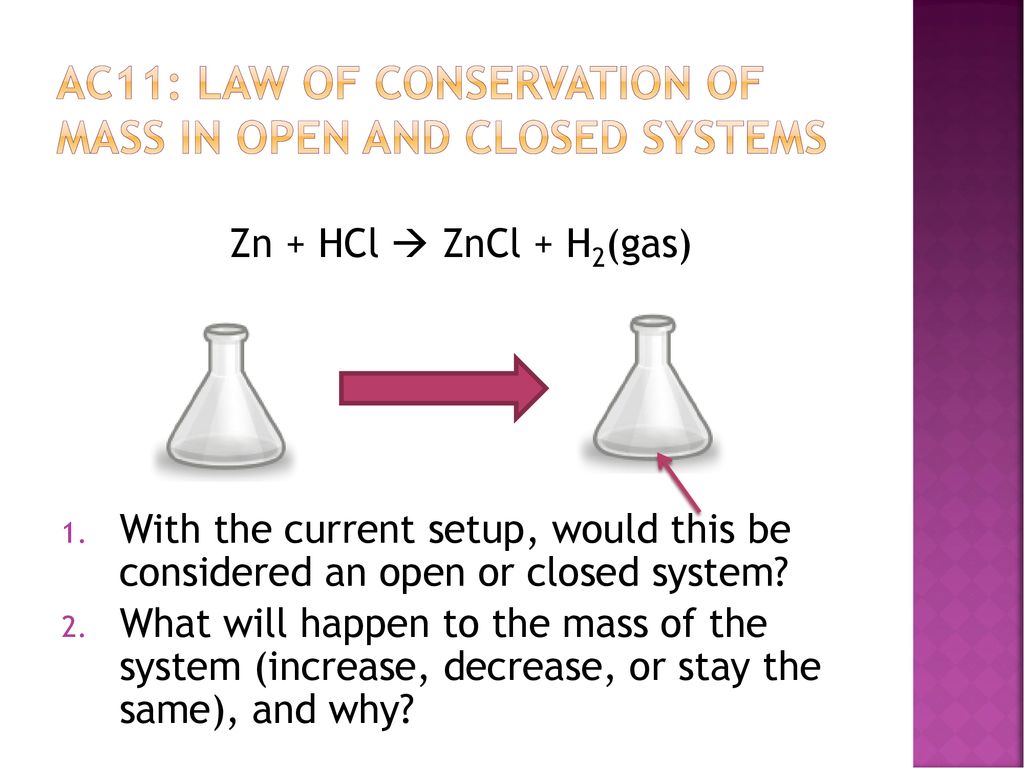 Conservation Of Mass In Open And Closed Systems Ppt Download
Conservation Of Mass In Open And Closed Systems Ppt Download
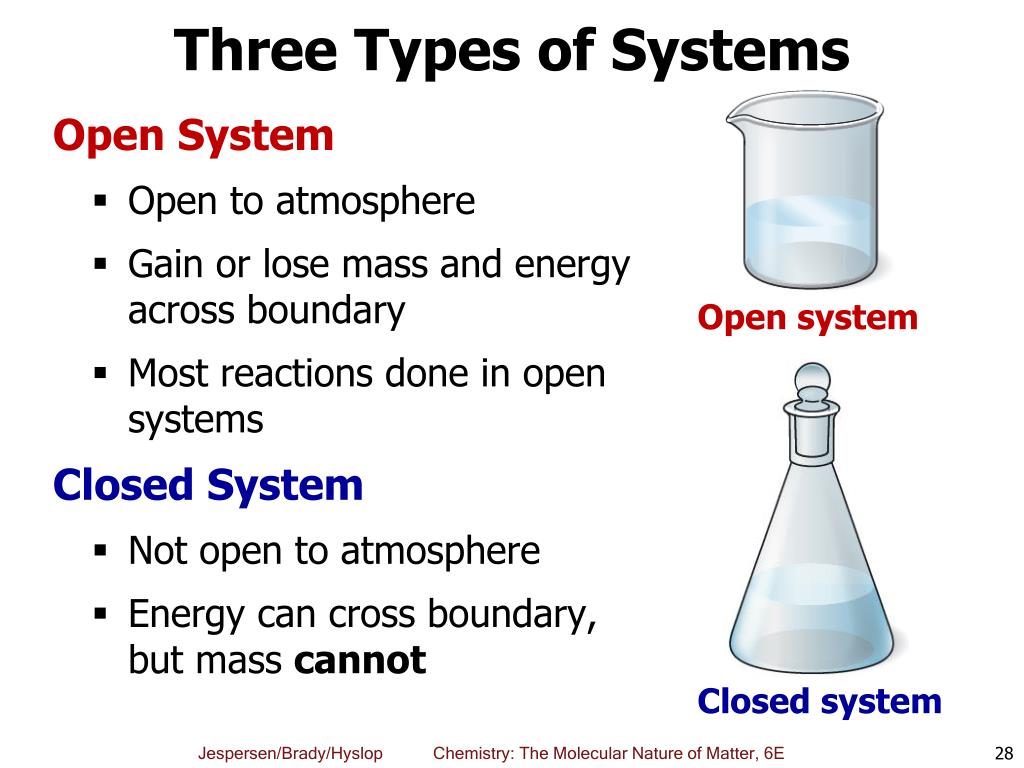 Ppt Chapter 7 Energy And Chemical Change Powerpoint
Ppt Chapter 7 Energy And Chemical Change Powerpoint
 Closed Open Systems Definition Examples Video Lesson
Closed Open Systems Definition Examples Video Lesson
Thermodynamic Systems Chemistry Community
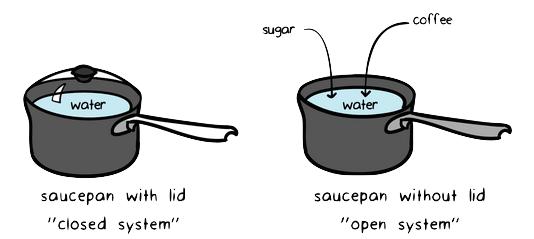 Thermodynamics Article Article Khan Academy
Thermodynamics Article Article Khan Academy
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcsxcrg9eeh3tlejsj1uhgtsuwg2 M Kedx 3tpkygqne3rs4aq Usqp Cau
 A System And Its Surroundings Chemistry Libretexts
A System And Its Surroundings Chemistry Libretexts
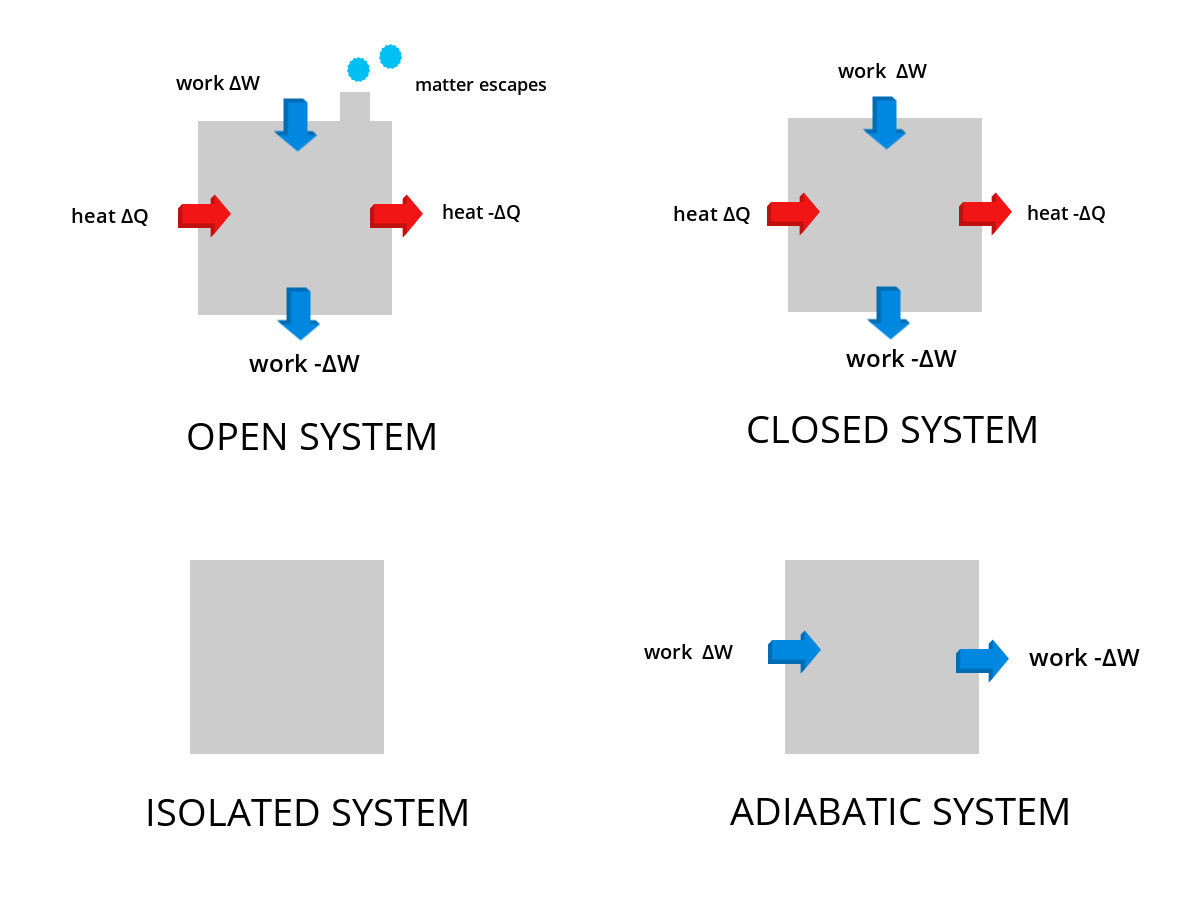 Themodynamic Systems Open Closed Isolated Systems
Themodynamic Systems Open Closed Isolated Systems
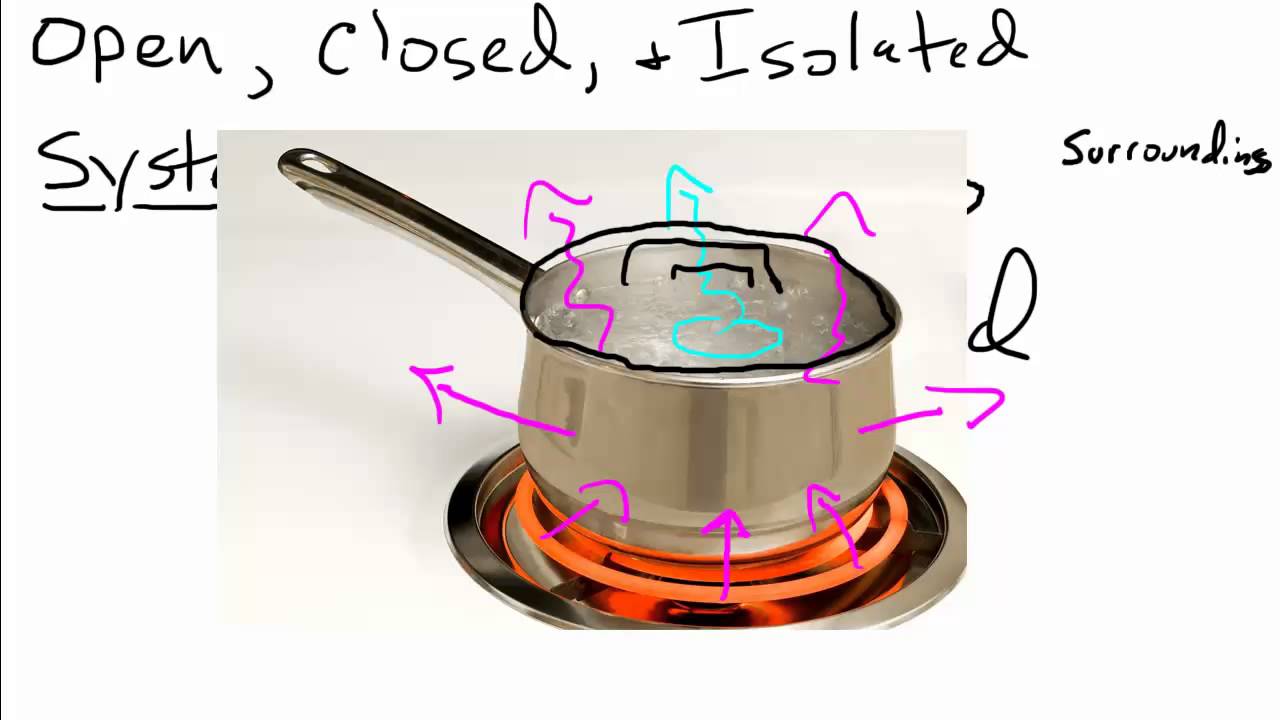 Open Closed Isolated Systems Youtube
Open Closed Isolated Systems Youtube
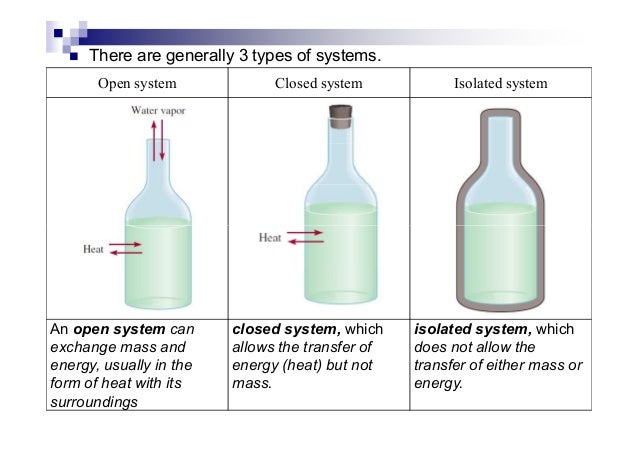 Inorganic Chemistry Thermochemistry
Inorganic Chemistry Thermochemistry

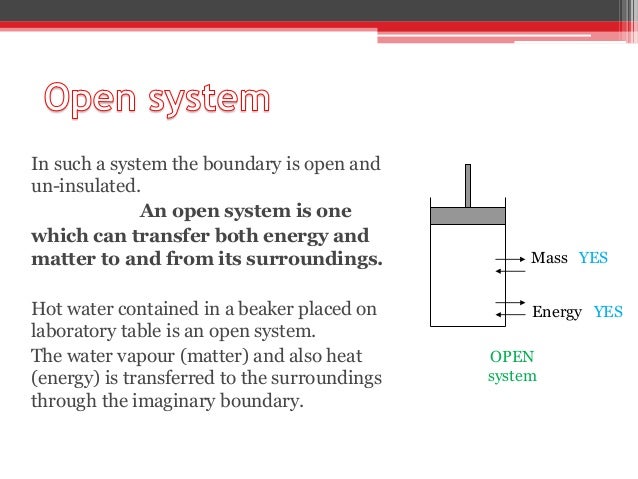 Diploma I Applied Science Chemistry U V Thermodynamics
Diploma I Applied Science Chemistry U V Thermodynamics
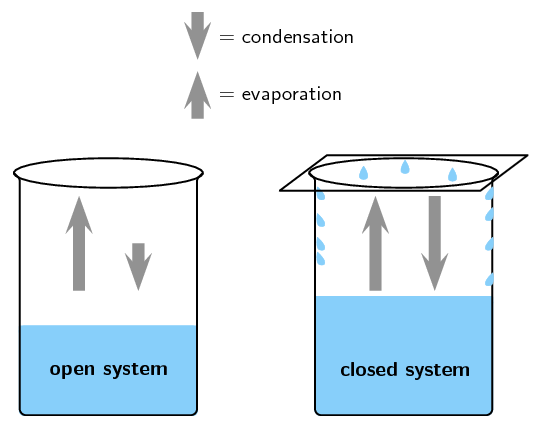 Open And Closed Systems Chemical Equilibrium
Open And Closed Systems Chemical Equilibrium
 File Open Closed And Isolated Systems Png Wikimedia Commons
File Open Closed And Isolated Systems Png Wikimedia Commons
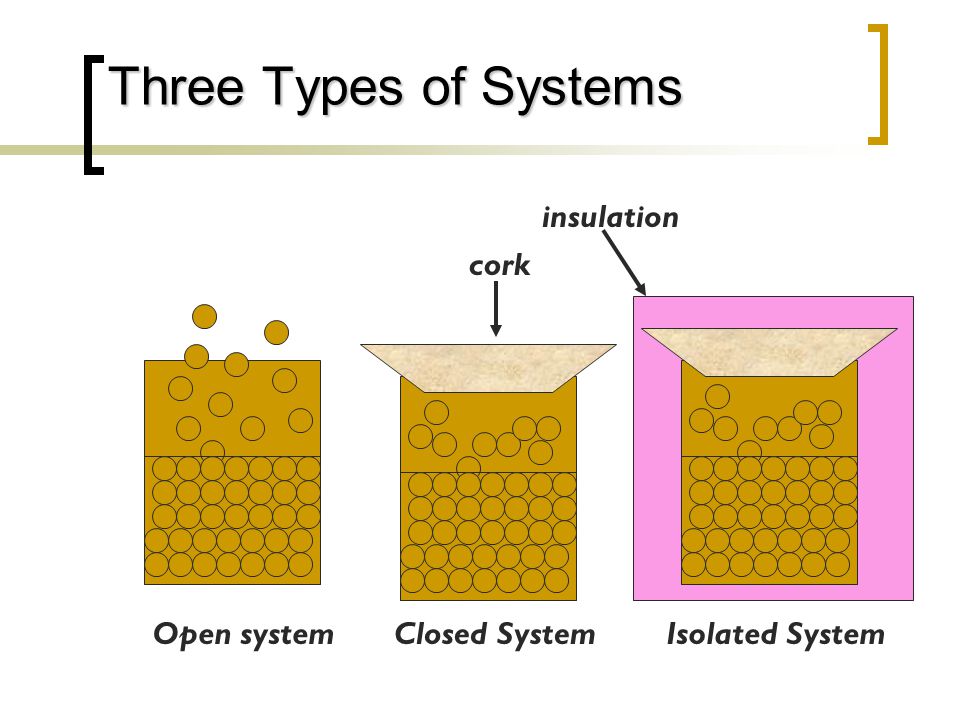 Energy Relationships In Chemistry Ppt Video Online Download
Energy Relationships In Chemistry Ppt Video Online Download
Http Coralgables Sh Enschool Org Ourpages Auto 2016 7 21 33705188 Lecture 2019 20 20topic 205 20part 201 20slides Pdf
Systems Physical Chemistry Of Earth S Materials
 Open Closed And Isolated Systems In Chemistry
Open Closed And Isolated Systems In Chemistry
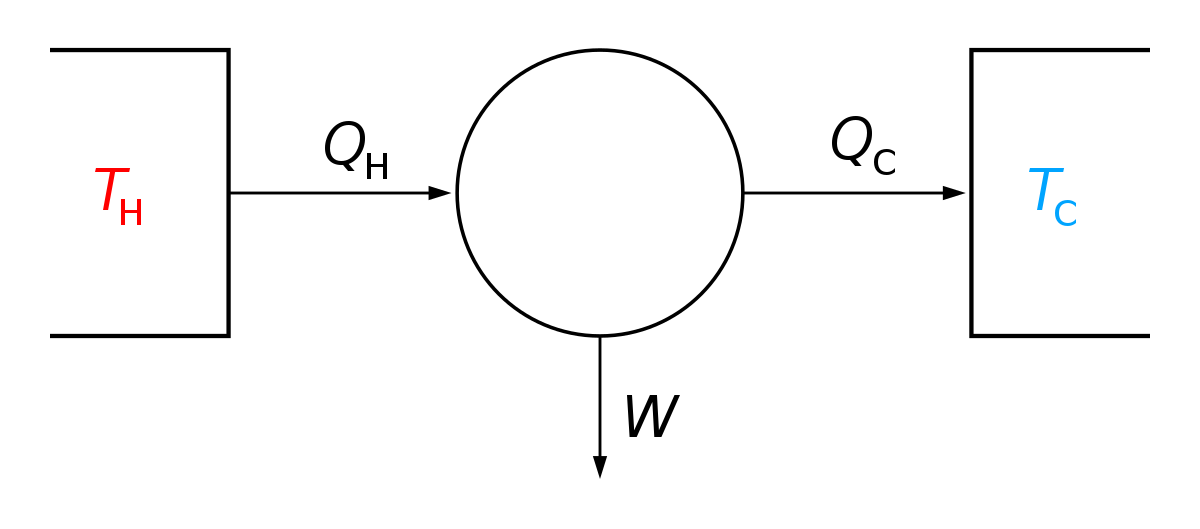 First Law Of Thermodynamics Wikipedia
First Law Of Thermodynamics Wikipedia
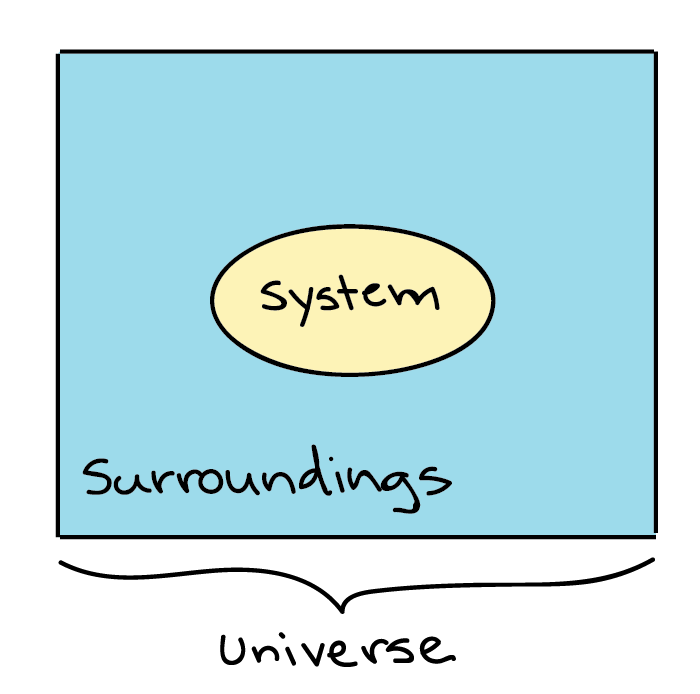 The Laws Of Thermodynamics Article Khan Academy
The Laws Of Thermodynamics Article Khan Academy
 A System And Its Surroundings Chemistry Libretexts
A System And Its Surroundings Chemistry Libretexts
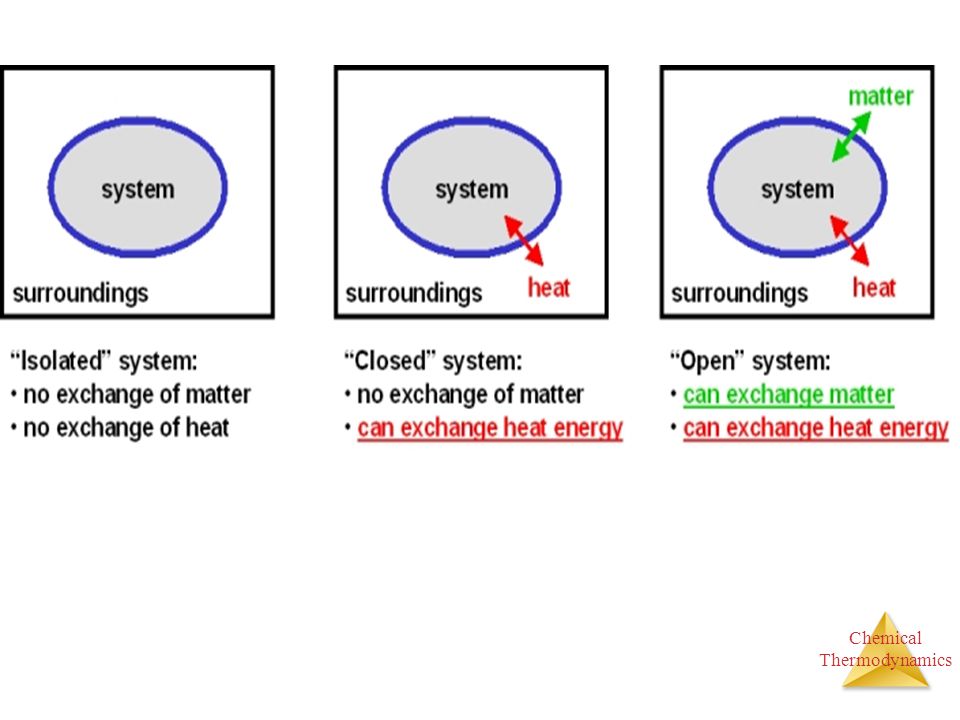 Chemical Thermodynamics Lecture 1 Chemical Thermodynamics
Chemical Thermodynamics Lecture 1 Chemical Thermodynamics
Difference Between Open And Closed System Definition
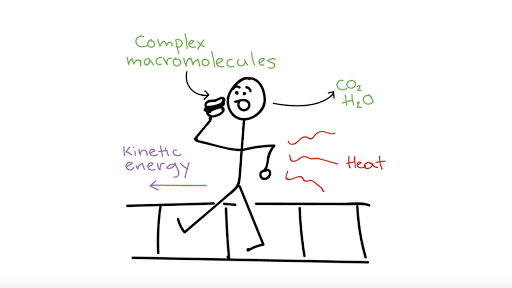
 A System And Its Surroundings Chemistry Libretexts
A System And Its Surroundings Chemistry Libretexts
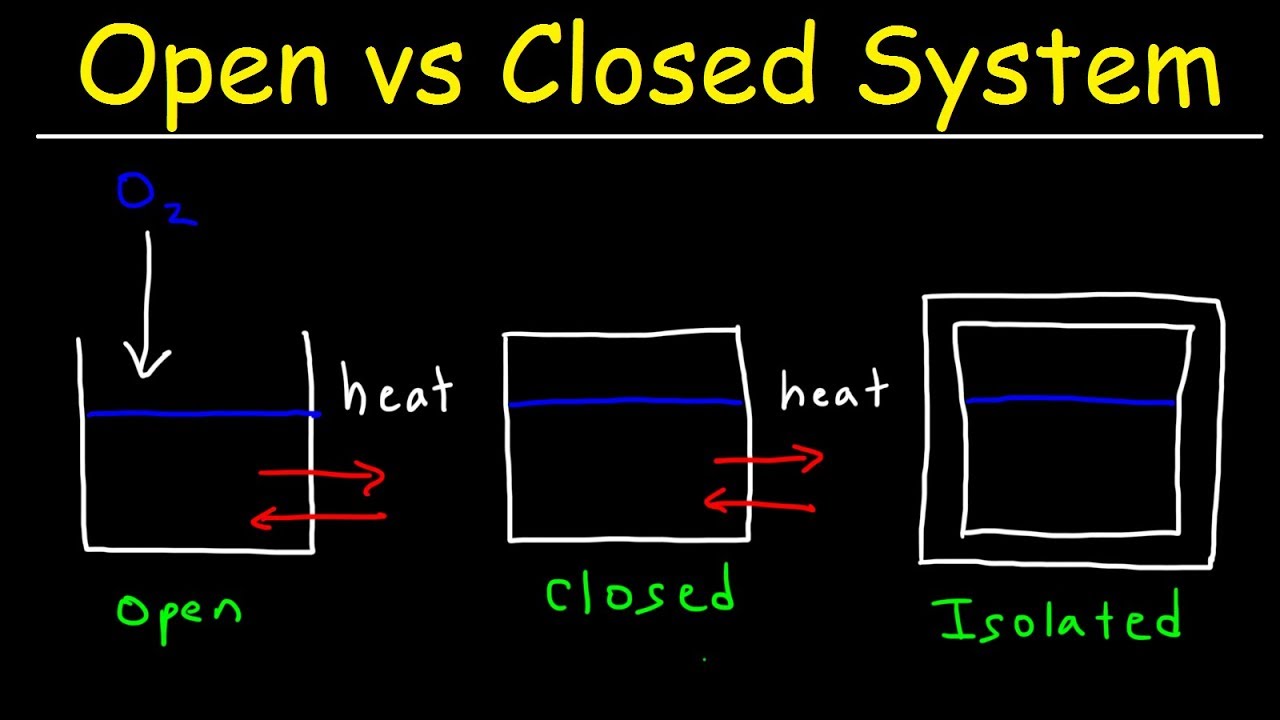 Open System Closed System And Isolated System Thermodynamics
Open System Closed System And Isolated System Thermodynamics
 A System And Its Surroundings Chemistry Libretexts
A System And Its Surroundings Chemistry Libretexts
 Chemical Reactions Ppt Video Online Download
Chemical Reactions Ppt Video Online Download
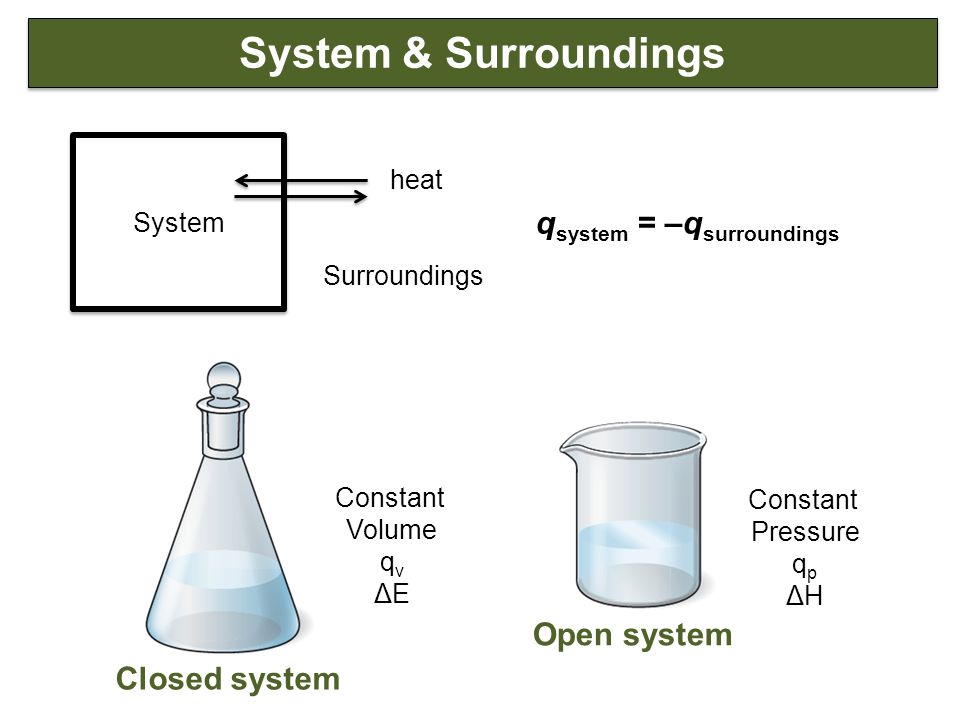 Review Chapter 6 Oxidation Reduction Reactions Chemistry The
Review Chapter 6 Oxidation Reduction Reactions Chemistry The
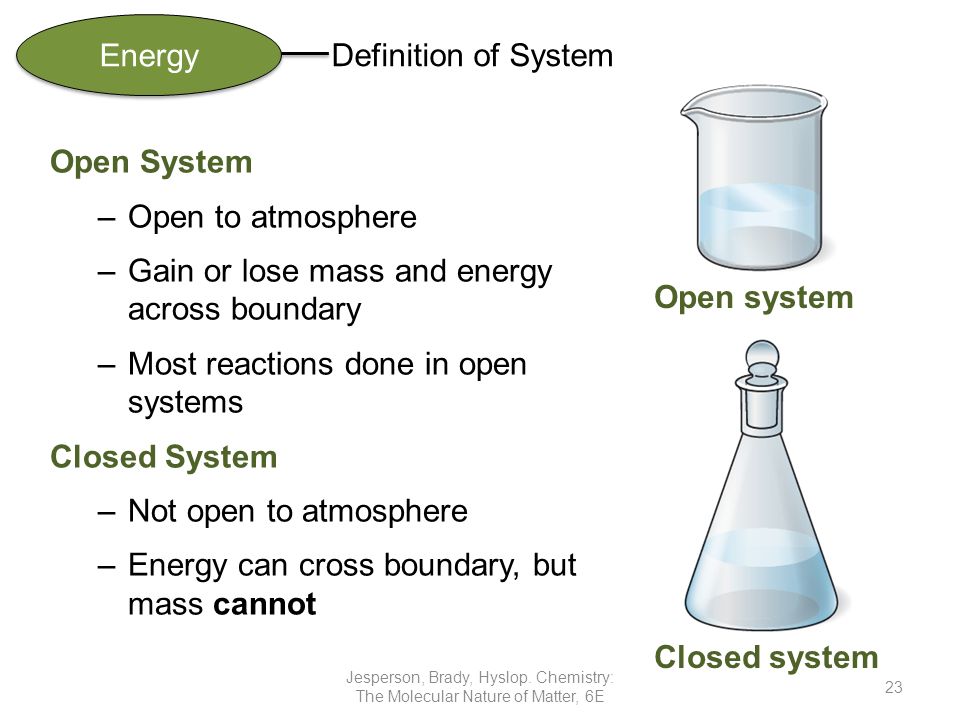 Energy Chemical Change Chapter 7 Chemistry The Molecular Nature
Energy Chemical Change Chapter 7 Chemistry The Molecular Nature
 A System And Its Surroundings Chemistry Libretexts
A System And Its Surroundings Chemistry Libretexts

Posting Komentar
Posting Komentar