Which Are Intensive Properties
Specific properties of material are derived from other intensive and extensive properties of that material. Silver gold and copper are excellent conductors of electricity while glass and plastic are poor conductors.
Is Melting Point Intensive Or Extensive
The extensive properties scale directly with size i e.

Which are intensive properties. For example the density of water is an intensive property and can be derived from measurements of the mass of a water volume an extensive property divided by the volume another extensive property. Intensive properties show the same result in different samples test whereas extensive properties show the variable result in the different samples test. For example volume is an extensive property.
Intensive properties are those that do not change as the size of an object changes. Or intrinsic they do not depend on the amount of matter ie they remain unchanged. If the system is divided by a wall that is permeable to heat or to matter the temperature of each subsystem is identical.
A larger or smaller piece of glass will not change this property. In contrast an extensive property is one that does depend on sample size. An intensive property is one that does not depend on the mass of the substance or system.
Here s a look at what intensive and extensive properties are examples of them and how to tell them apart. Intensive properties and extensive properties are types of physical properties of matter. The ratio of two extensive properties however is an intensive property e g density is mass per unit volume.
For example the temperature of a system in thermal equilibrium is the same as the temperature of any part of it. If the size of a system doubles the value of an extensive property simply doubles as well. The electrical conductivity of a substance is a property that depends only on the type of substance.
Examples of extensive properties include mass and volume. Intensive properties are intrinsic or essential to the nature of the substance. There are some extensive properties that can be used as intensive.
Also heat capacity which is an extensive property of a system. The properties of matter that do not depend on the size or quantity of matter in any way are referred to as an intensive property of matter. The terms intensive and extensive were first described by physical chemist and physicist richard c.
Intensive properties are boiling point color the state of matter density odor melting point hardness malleability whereas extensive properties include mass volume length height etc. An intensive property is a physical quantity whose value does not depend on the amount of the substance for which it is measured. Intensive properties on the other hand would simply remain constant whether the.
Temperature t pressure p and density r are examples of intensive properties. We re going to look into a few examples to better understand these distinctions. Extensive properties are those that change as the size of an object changes.
 Under What Condition Extensive Property Became Intensive Property Quora
Under What Condition Extensive Property Became Intensive Property Quora
/intensive-vs-extensive-properties-604133-v3-5b55fb394cedfd0037117796.png) The Difference Between Intensive And Extensive Properties
The Difference Between Intensive And Extensive Properties
 Unit 2 Lesson 2 1 Properties Of Matter Part One
Unit 2 Lesson 2 1 Properties Of Matter Part One
 Thermodynamics Intensive And Extensive Properties Intensive Properties System Properties Whose Magnitudes Are Independent Of The Total Amount Instead Ppt Download
Thermodynamics Intensive And Extensive Properties Intensive Properties System Properties Whose Magnitudes Are Independent Of The Total Amount Instead Ppt Download
Difference Between Intensive And Extensive Properties Definition Examples And Differences
 Properties Of Matter Extensive And Intensive Properties Presentation Youtube
Properties Of Matter Extensive And Intensive Properties Presentation Youtube
 Extensive Vs Intensive Properties Chemistry Classroom Chemistry Class Chemistry
Extensive Vs Intensive Properties Chemistry Classroom Chemistry Class Chemistry
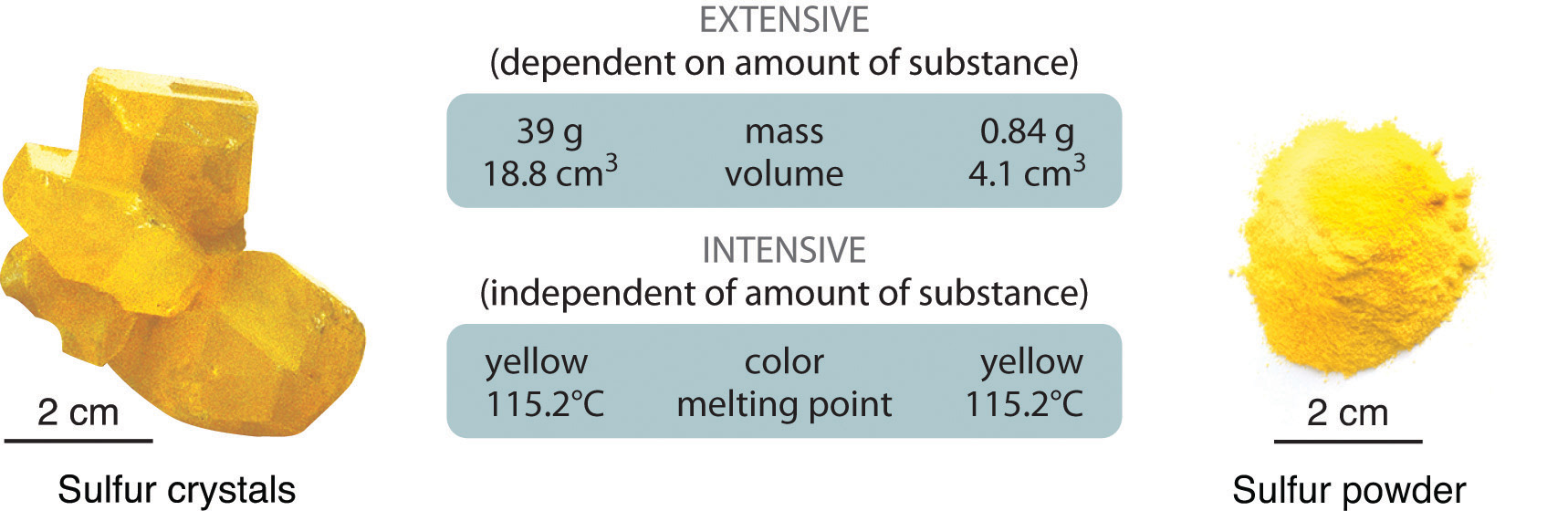 1 3 Properties Of Matter Chemistry Libretexts
1 3 Properties Of Matter Chemistry Libretexts
 What Is The Difference Between Extensive And Intensive Properties Proprofs Discuss
What Is The Difference Between Extensive And Intensive Properties Proprofs Discuss
Difference Between Intensive And Extensive Properties Intensive Vs Extensive Properties
 Examples Of Extensive And Intensive Properties Download Table
Examples Of Extensive And Intensive Properties Download Table
 Extensive Intensive Properties Ppt Download
Extensive Intensive Properties Ppt Download

 What Are Intensive And Extensive Properties The Intelligence Band
What Are Intensive And Extensive Properties The Intelligence Band
Difference Between Intensive Property And Extensive Property
 Extensive And Intensive Properties
Extensive And Intensive Properties
Examples Of Intensive Extensive Properties Of Matter Video Lesson Transcript Study Com
 Thermodynamics And Fluid Mechanics C1 L2 Intensive And Extensive Properties 1 Youtube
Thermodynamics And Fluid Mechanics C1 L2 Intensive And Extensive Properties 1 Youtube
Physical And Chemical Properties And Changes Lessons Tes Teach
 Intensive Vs Extensive Properties Youtube
Intensive Vs Extensive Properties Youtube
 Why Pressure And Density Are Intensive Property Thermodynamics Youtube
Why Pressure And Density Are Intensive Property Thermodynamics Youtube
 Properties Of Matter Physical Chemical Chemistrygod
Properties Of Matter Physical Chemical Chemistrygod
 Intensive Vs Extensive Properties Physical And Chemical Properties Chemistry Basics Chemistry Classroom
Intensive Vs Extensive Properties Physical And Chemical Properties Chemistry Basics Chemistry Classroom
What Do You Mean By Intensive And Extensive Properties Of Matter Quora
2 1 Properties Of Matter Chemistry
 Density An Intensive Property What Are Extensive And Intensive Properties Property Refers To The Characteristics Of A Material Property Refers To Ppt Download
Density An Intensive Property What Are Extensive And Intensive Properties Property Refers To The Characteristics Of A Material Property Refers To Ppt Download
 Solved Classify The Following Parameters As Intensive Pro Chegg Com
Solved Classify The Following Parameters As Intensive Pro Chegg Com
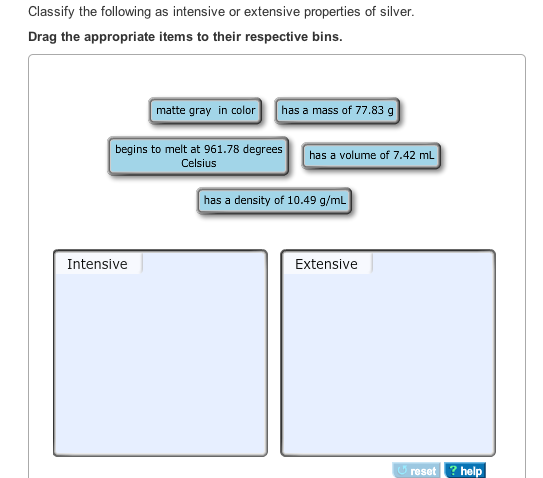 Solved Classify The Following As Intensive Or Extensive P Chegg Com
Solved Classify The Following As Intensive Or Extensive P Chegg Com
 Thermodynamic Equilibrium Intensive And Extensive Properties Mechanical Engineering Concepts And Principles
Thermodynamic Equilibrium Intensive And Extensive Properties Mechanical Engineering Concepts And Principles
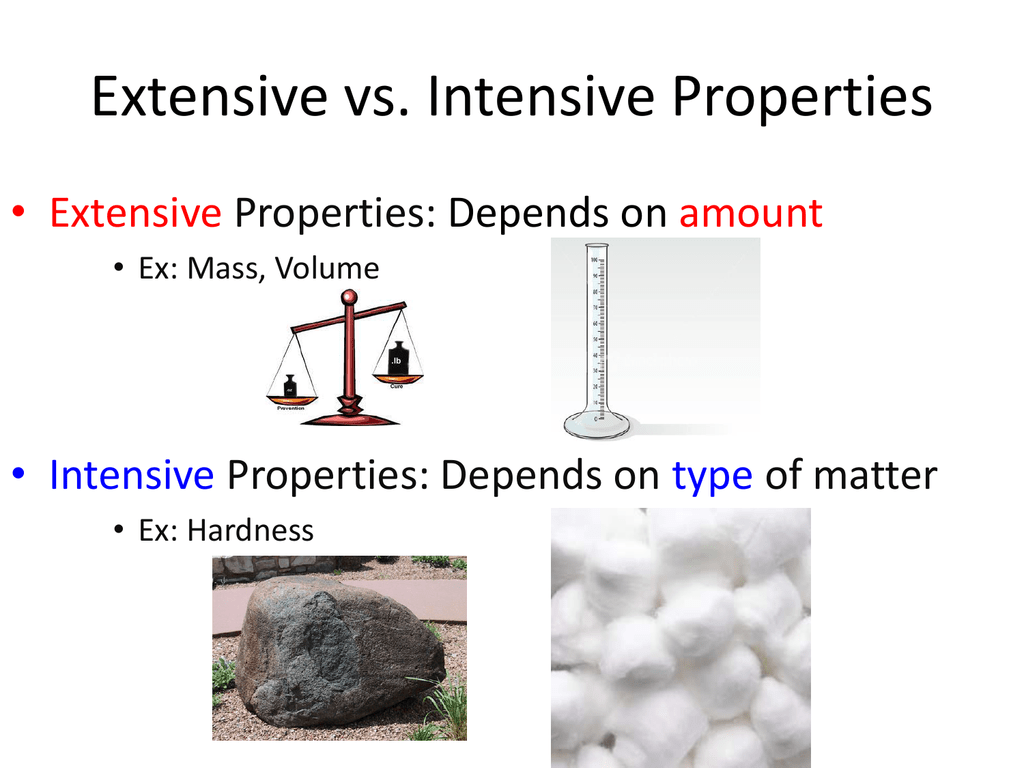 Extensive Vs Intensive Properties
Extensive Vs Intensive Properties
Https Www Imater Org Ourpages Auto 2018 8 22 58546062 Matter Pdf
 Intensive Extensive Properites Youtube
Intensive Extensive Properites Youtube

 3 5 Intensive And Extensive Properties Youtube
3 5 Intensive And Extensive Properties Youtube
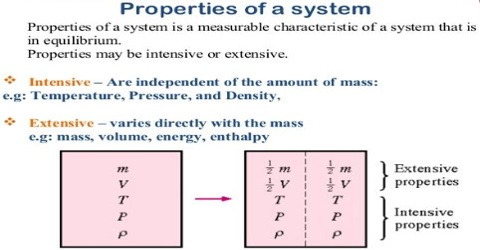 Intensive And Extensive Thermodynamic Properties Qs Study
Intensive And Extensive Thermodynamic Properties Qs Study
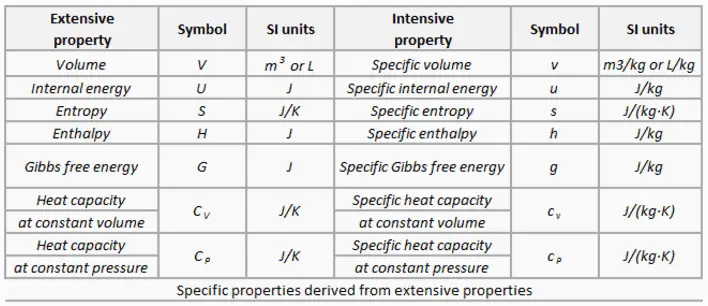 What Is Extensive And Intensive Property Definition
What Is Extensive And Intensive Property Definition
:max_bytes(150000):strip_icc()/close-up-view-diamond-705350873-59df98d4054ad9001156f228.jpg)

Posting Komentar
Posting Komentar