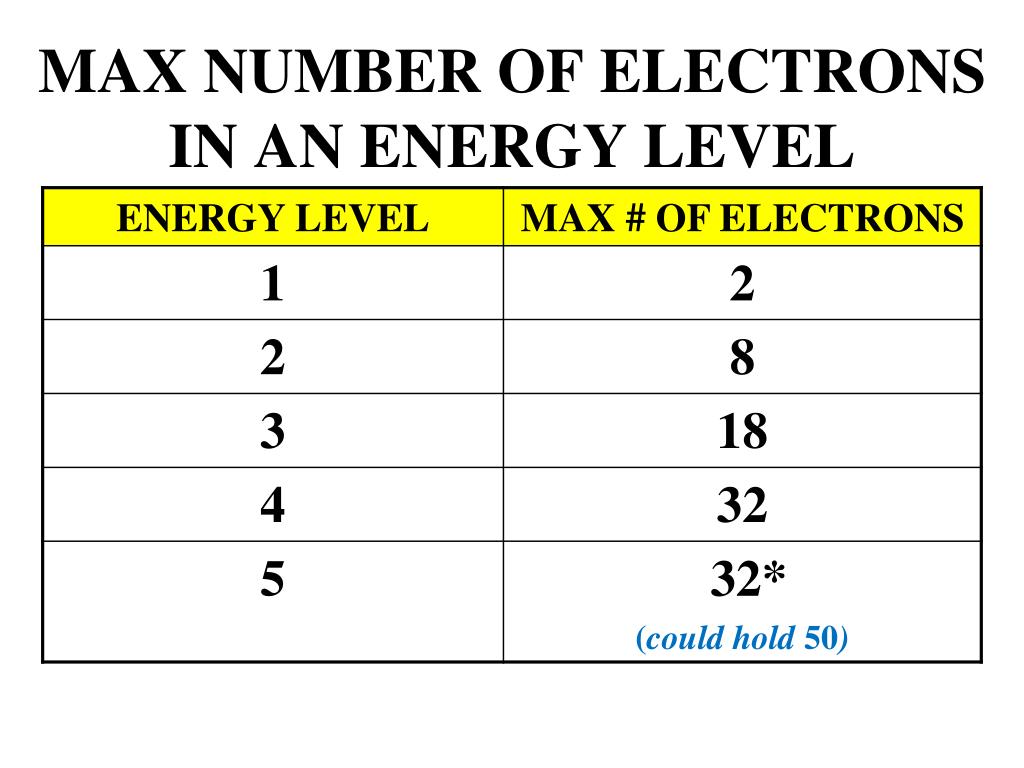
Maximum Number Of Electrons In Each Energy Level
This table shows the pattern in the periodic table that mendeleev developed and how the missing elements at that time could be predicted. Following the formula the third energy level can contain 18 electrons the fourth energy level can hold 32 electrons the fifth energy level can hold 50 electrons the sixth energy level can carry up to 72 electrons and the seventh energy.
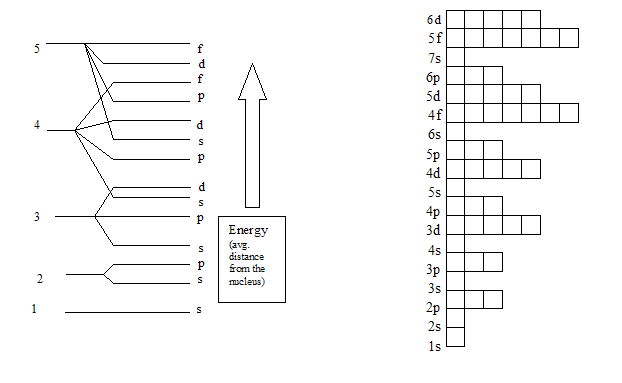 Summary Of Electron Configurations
Summary Of Electron Configurations
Claim your free seat in vedantu master classes.
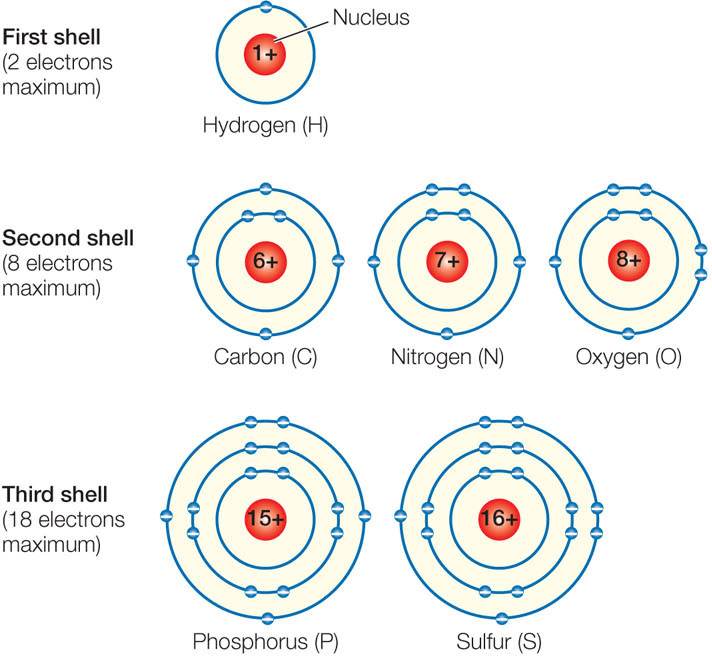
Maximum number of electrons in each energy level. Below is a table showing the maximum number of electrons an element can have for each of its energy level shells. The maximum number of electrons that an energy level can hold is determined from the formula 2n 2 equals the total number where n is the energy level. The first shell can hold up to two electrons the second shell can hold up to eight 2 6 electrons the third shell can hold up to 18 2 6 10 and so on.
Electron capacity 2 n2. Principal quantum number shell letter. The information shown is for elements with atomic numbers 1 to 20.
Each shell can contain only a fixed number of electrons. Thus the first energy level holds 2 1 2 2 electrons while the second holds 2 2 2 8 electrons. The energy levels are typically referred to by their shell number rather than their energy level.
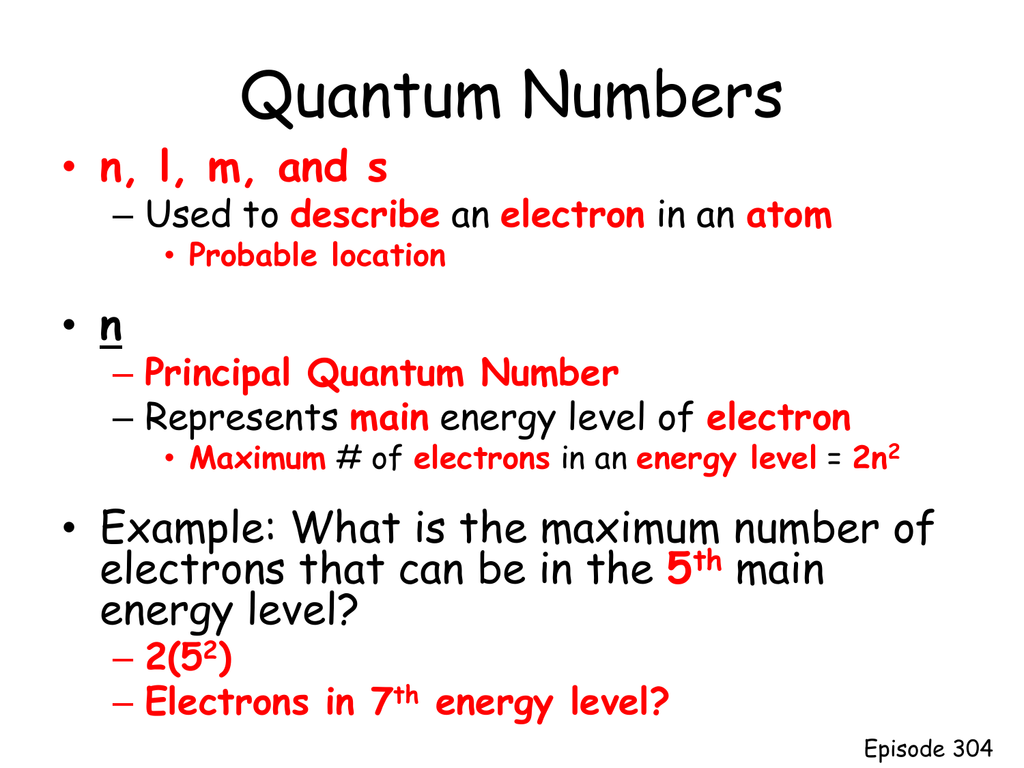
Thus the third level holds a maximum of 18 electrons. To calculate the maximum number of electrons in each energy level the formula 2 n2 can be used where n is the principal energy level first quantum number. The maximum number of electrons found on energy levels one through six are two eight 18 32 50 and 72.
For example energy level 1 2 1 2 calculates to two possible electrons that will fit into the first energy level. The five d orbitals can hold up to 10 electrons. The electronic configuration and the number of electrons in each energy level of chlorine is 2 8 7 how many electrons belong in each energy level.
The general formula is that the n th shell can in principle hold up to 2 n2 electrons. The fourth and higher levels also have an f sublevel containing seven f orbitals which can hold a maximum of 14 electrons. The first energy level k contains 2 electrons second energy level 8 third energy level 18 and fourth energy level has 32 electrons.
2 in the s orbital 6 in the three p orbitals and 10 in the five d orbitals. The maximum number of electrons that can occupy a specific energy level can be found using the following formula. The variable n represents the principal quantum number the number of the energy level in question.
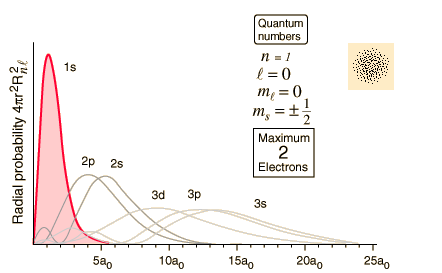
Electrons orbit the atom s nucleus in energy levels. The formula for determining the number of electrons is two multiplied by n squared or 2n 2.
What Are The Maximum Number Of Electrons In Each Shell Quora
 How Are Electrons Distributed In Different Orbits Electronic
How Are Electrons Distributed In Different Orbits Electronic
 Ppt Fact Powerpoint Presentation Free Download Id 3878389
Ppt Fact Powerpoint Presentation Free Download Id 3878389
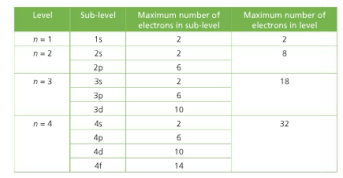 8 The Main Energy Level Or Shell Is Given An Integer Number N
8 The Main Energy Level Or Shell Is Given An Integer Number N
 Electrons Biology For Majors I
Electrons Biology For Majors I
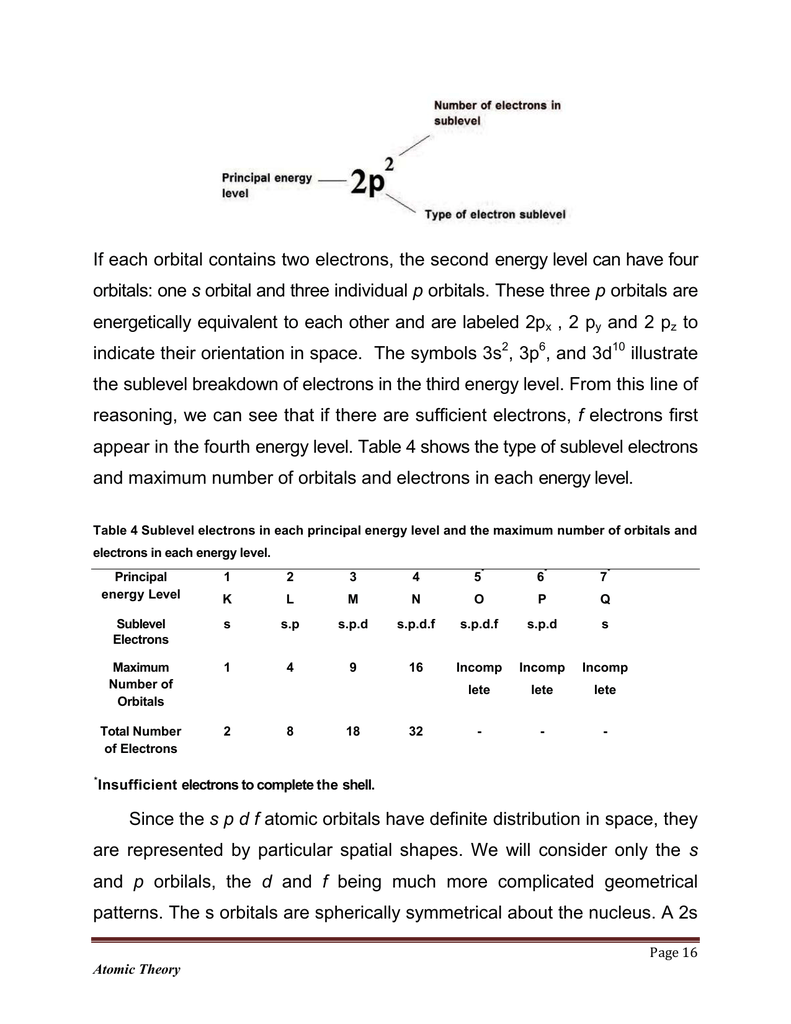 If Each Orbital Contains Two Electrons The Second Energy Level Can
If Each Orbital Contains Two Electrons The Second Energy Level Can
Main Energy Levels Or Shells Sublevels Or Subshells
 How To Determine The Maximum Number Of Electrons Using Allowed
How To Determine The Maximum Number Of Electrons Using Allowed
 Solved 8 What Is The Maximum Number Of Electrons That Ca
Solved 8 What Is The Maximum Number Of Electrons That Ca
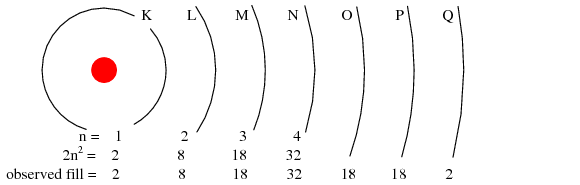 Maximum Number Of Electron In N Energy Level Sutori
Maximum Number Of Electron In N Energy Level Sutori
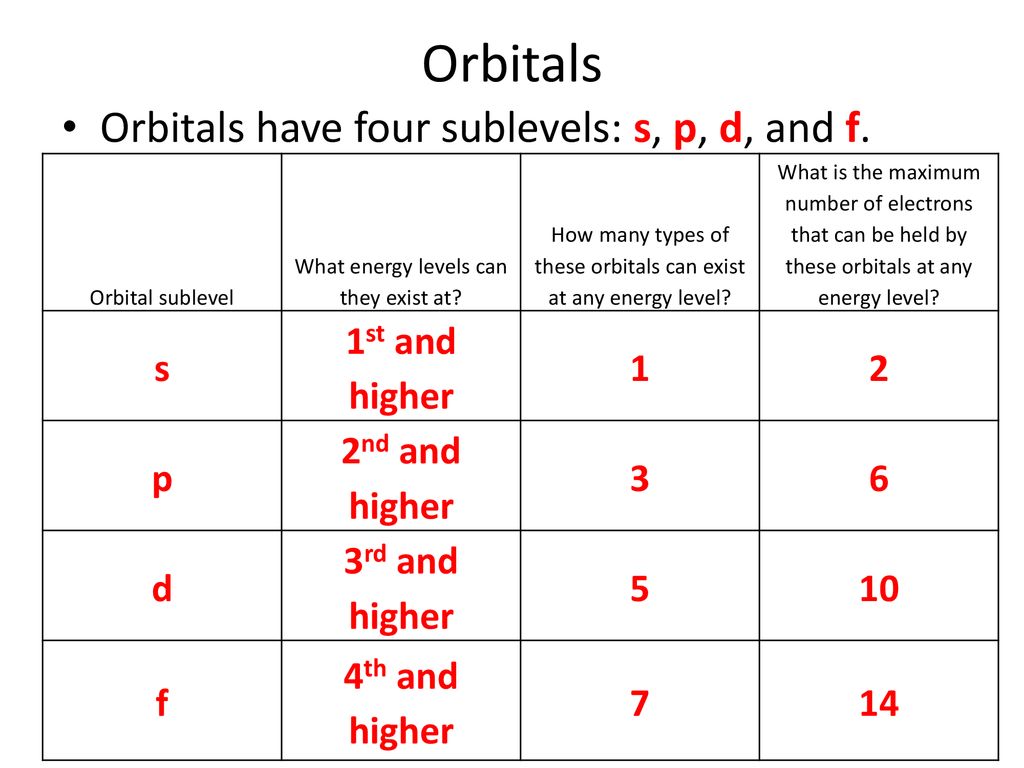 Electron Configuration Guided Notes Ppt Download
Electron Configuration Guided Notes Ppt Download
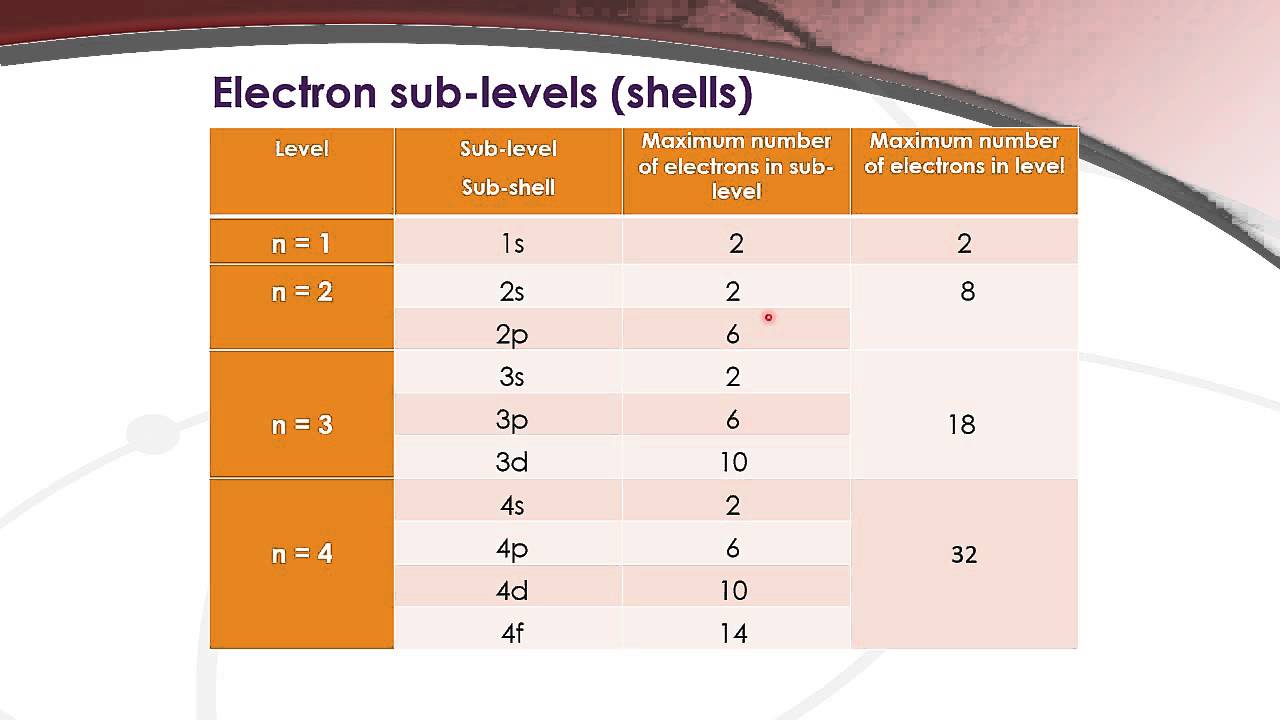 12 1 4 State The Maximum Number Of Orbitals In A Given Energy
12 1 4 State The Maximum Number Of Orbitals In A Given Energy
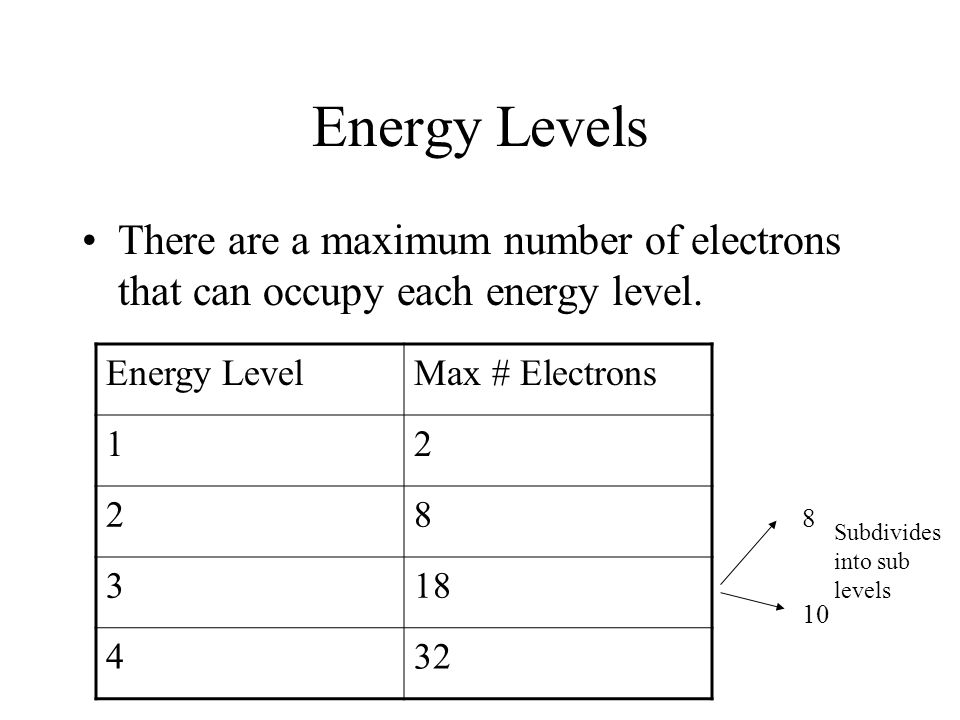 Valence Electrons Ppt Video Online Download
Valence Electrons Ppt Video Online Download
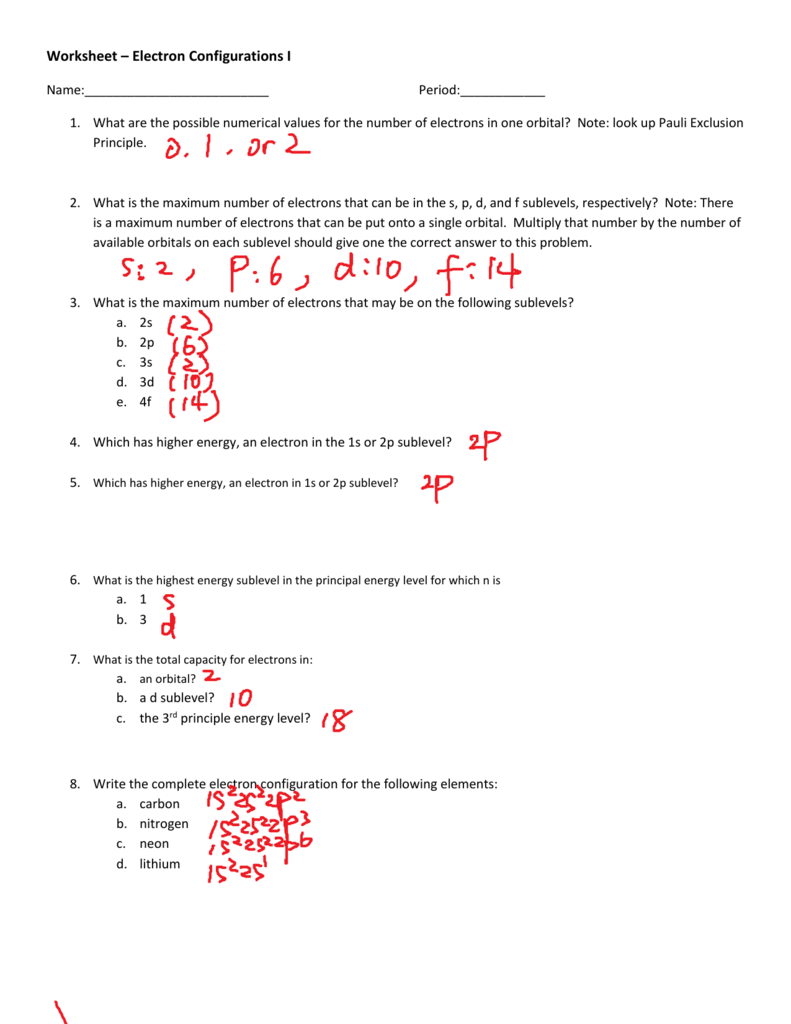 Electron Configurations Worksheet I Answers
Electron Configurations Worksheet I Answers
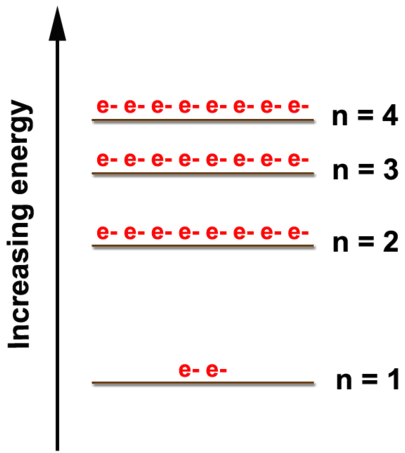 2 4 Electron Arrangements Chemistry Libretexts
2 4 Electron Arrangements Chemistry Libretexts
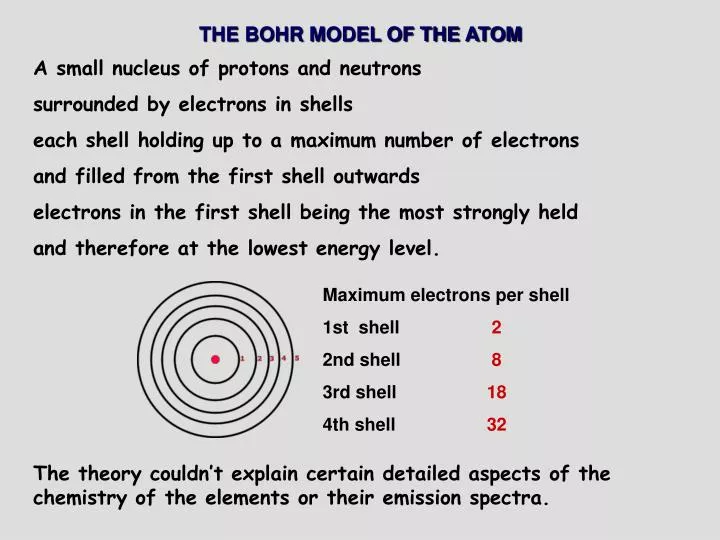 Ppt The Bohr Model Of The Atom Powerpoint Presentation Free
Ppt The Bohr Model Of The Atom Powerpoint Presentation Free

 Orbital Model Electron Cloud Model The Energy Levels Of Electrons
Orbital Model Electron Cloud Model The Energy Levels Of Electrons
 The Pauli Exclusion Principle Physics
The Pauli Exclusion Principle Physics
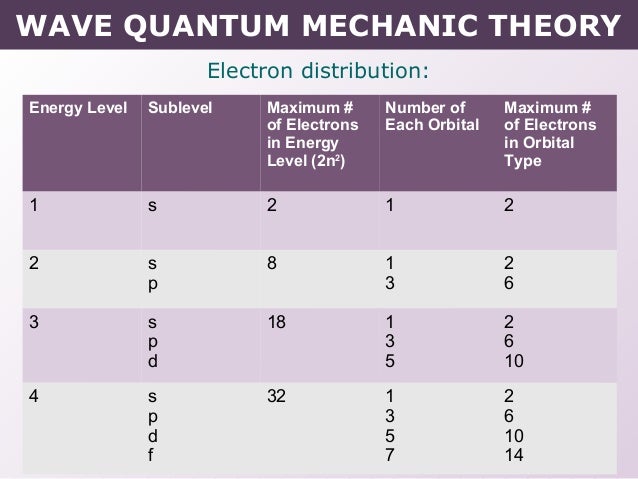 Tang 02 Wave Quantum Mechanic Model
Tang 02 Wave Quantum Mechanic Model
 Solved A Fill The Following Blanks 1 Indicate The Maxim
Solved A Fill The Following Blanks 1 Indicate The Maxim
 What Are The Maximum Number Of Electrons In Each Shell
What Are The Maximum Number Of Electrons In Each Shell
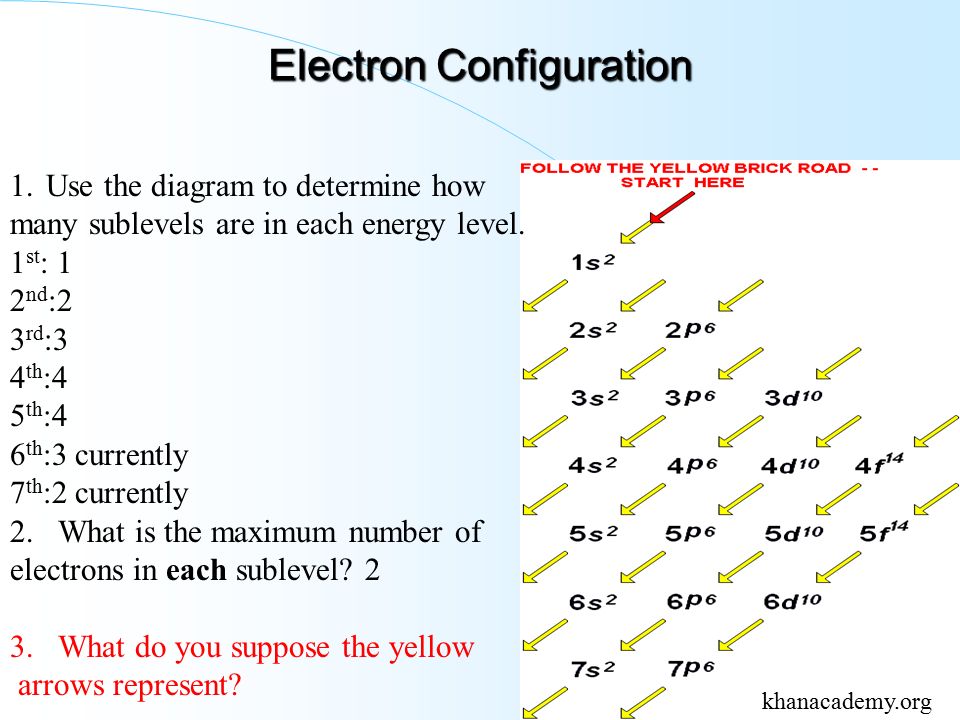 Electron Properties And Arrangement Chapter 5 Ppt Video Online
Electron Properties And Arrangement Chapter 5 Ppt Video Online
 Sci C Un5elctrncnfgrtnorbtldgrms
Sci C Un5elctrncnfgrtnorbtldgrms
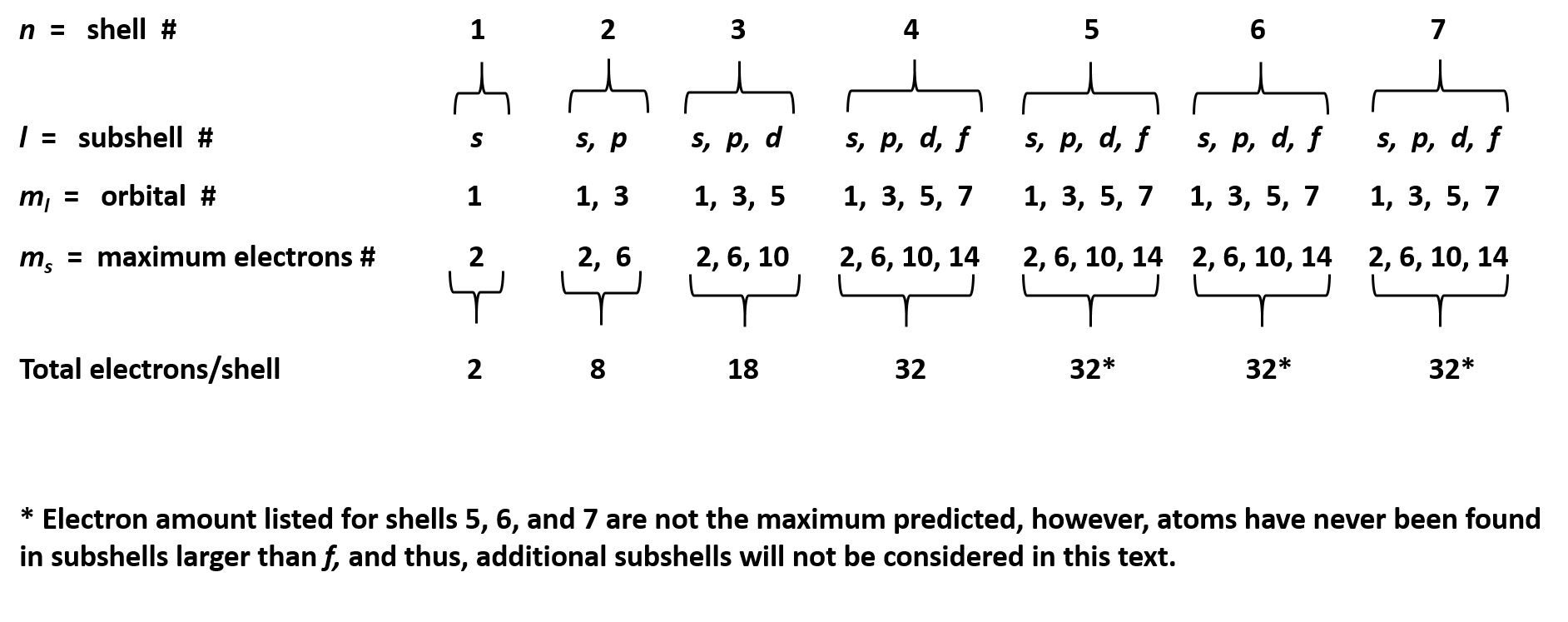 Ch150 Chapter 2 Atoms And Periodic Table Chemistry
Ch150 Chapter 2 Atoms And Periodic Table Chemistry
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctfoiis3q2rrf3p2 Jcgpgifx88fnqt6y5ktklrla0v Ismgxz3 Usqp Cau

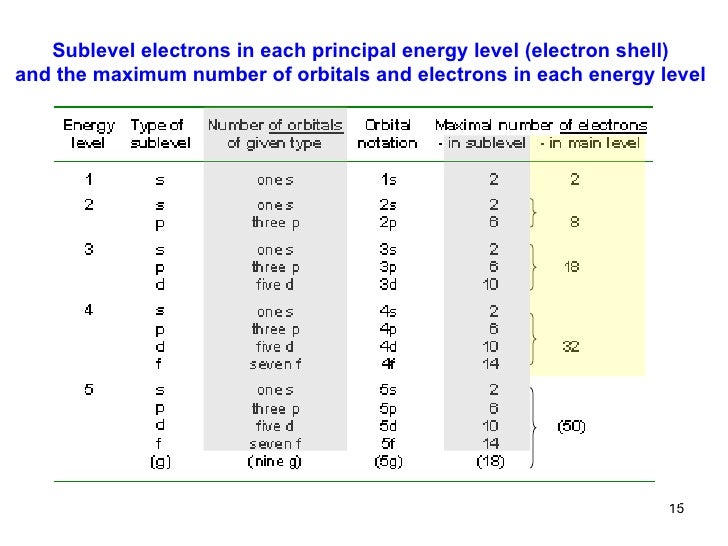



Posting Komentar
Posting Komentar