Difference Between Oxidation And Reduction
Oxidation and reduction are the two half reactions of redox reactions a redox reaction is a chemical reaction that occurs through the electron exchange between atoms. These are balanced chemical reactions.
 Difference Between Oxidation And Reduction Chemical Reactions And
Difference Between Oxidation And Reduction Chemical Reactions And
For many students the confusion occurs when attempting to identify which reactant was oxidized and which reactant was reduced.
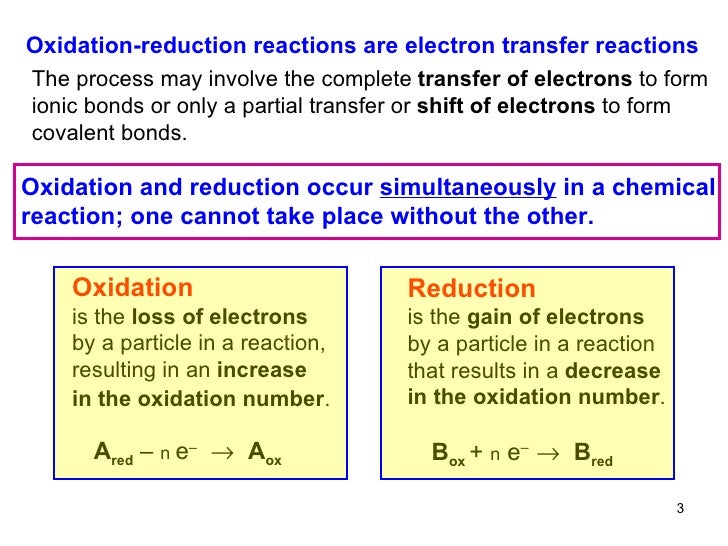
Difference between oxidation and reduction. When reduction oxidation reactions occur simultaneously it is called as redox reaction or reduction oxidation reaction. A reaction that has both oxidation and reduction within it is called a redox reaction. When molecules react and this reaction causes them to gain or lose electrons it is said that a redox reaction has taken place.
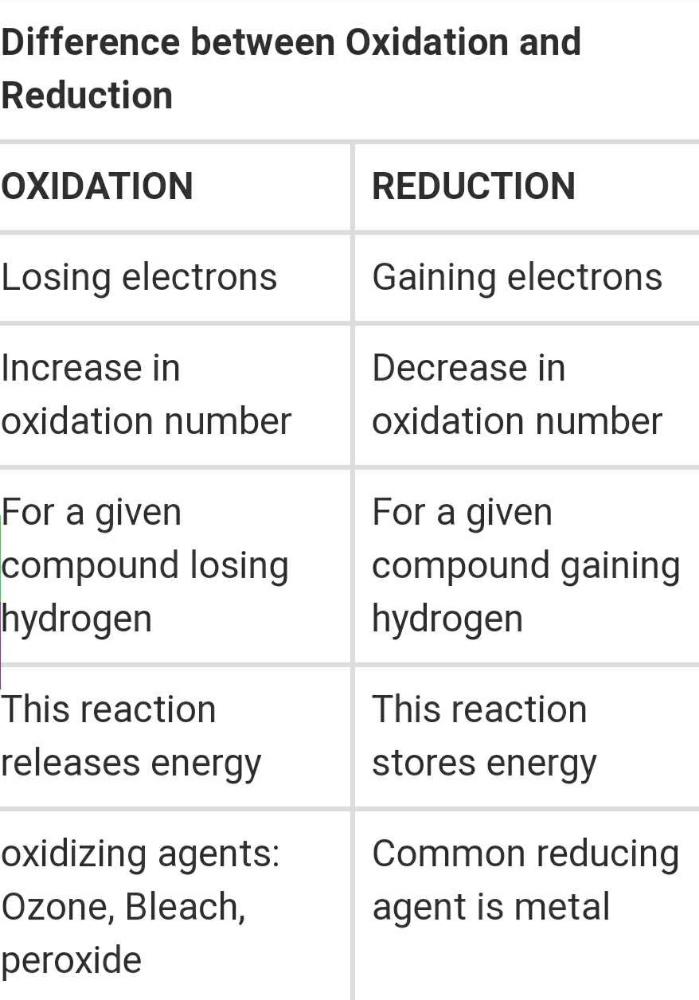
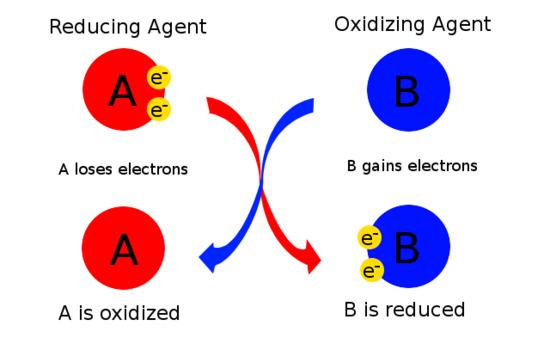
Oxidation can be well defined as the loss of electrons from a molecule atom or an ion whereas reduction is the gain of electrons from a molecule atom or an ion. Oxidation and reduction in terms of firing schedules electric kilns are naturally in an oxidation or neutral atmosphere. Oxidation vs reduction in organic and inorganic chemistry there are many chemical reactions that take place when two molecules are introduced to one another.
We can write redox reactions as half equations this can make it. Chemical reactions between different compounds are called redox reactions if the oxidation states of reactants are different from those of products. In oxidation reactions electrons are lost but in reduction reactions electrons are gained.
In chemistry if a substance is oxidised it loses electrons in a reaction. The main difference between oxidation and reduction is that oxidation is the increase of oxidation state whereas reduction is the decrease of oxidation state. What is the difference between oxidation and reduction.
In oxidation reactions oxygens are gained and in the reduction reactions oxygens are lost. What is the difference between oxidation reaction and reduction reaction. During this reaction there is an exchange of electrons.
If a substance is reduced it gains electrons in a reaction. The chemical reactions which often work together are oxidation and reduction. With fuel burning kilns however care must be taken to ensure that the kiln does not go into reduction until the latter part of the firing usually the last half hour to the last hour and a half.
The key difference between oxidation and reduction is that oxidation refers to the loss of electrons while reduction refers to the gain of electrons. The main difference between oxidation and reduction is that oxidation is the increasing of oxidation state of an atom whereas reduction is the decreasing of the oxidation state of an atom. Redox is short for reduction oxidation which is what occurs in any chemical reaction.
In oxidation hydrogen is lost but in reduction hydrogen is gained. Difference between oxidation and reduction. Main difference oxidation vs reduction.
Oxidation and reduction are two types of chemical reactions that often work together oxidation and reduction reactions involve an exchange of electrons between reactants.
 Difference Between Oxidation And Reduction Compare The
Difference Between Oxidation And Reduction Compare The
 1 Distinguish Between Oxidation And Reduction
1 Distinguish Between Oxidation And Reduction
 Give Two Differences Between Oxidation And Reduction Reactions B
Give Two Differences Between Oxidation And Reduction Reactions B
 How To Differentiate Between Oxidation And Reduction How To
How To Differentiate Between Oxidation And Reduction How To
 Difference Between At Least 3 Points With One Example 1
Difference Between At Least 3 Points With One Example 1
 Differentiate Between Oxidation And Reduction Edurev Class 10
Differentiate Between Oxidation And Reduction Edurev Class 10
 Oxidizing And Reducing Agents Chemistry Libretexts
Oxidizing And Reducing Agents Chemistry Libretexts
Difference Between Corrosion And Oxidation Definition Process
 Regent S Warm Up What Is The Electron Configuration Of A Sulfur
Regent S Warm Up What Is The Electron Configuration Of A Sulfur
 Oxidation And Reduction Video Khan Academy
Oxidation And Reduction Video Khan Academy
 Write The Differences Between Oxidation And Reduction Brainly In
Write The Differences Between Oxidation And Reduction Brainly In
 Redox Reaction Including Difference Between Reduction And
Redox Reaction Including Difference Between Reduction And
/rustyiron-58e69fde3df78c5162315571.jpg) What Is The Difference Between Oxidation And Reduction
What Is The Difference Between Oxidation And Reduction
Difference Between Nad And Nadp Definition Features Function
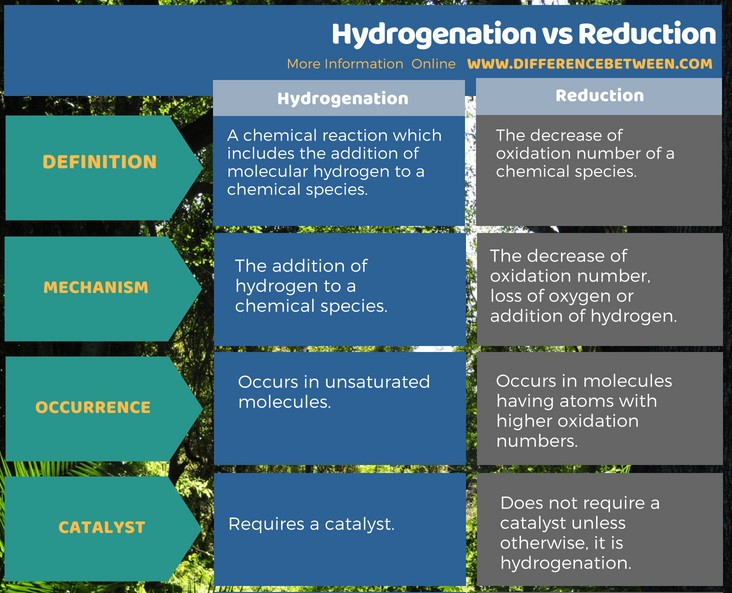
Difference Between Hydrogenation And Reduction Definition
 Topic 8 2 Cell Respiration Ppt Download
Topic 8 2 Cell Respiration Ppt Download
 Solved What Is The Difference Between Oxidation And Reduction
Solved What Is The Difference Between Oxidation And Reduction
 04 Redox Reactions Dissoln Precip
04 Redox Reactions Dissoln Precip
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcs X 6djgc42qm5yxlxd9k2pgij2tc2lw1hsg 4hczaovymwomx Usqp Cau
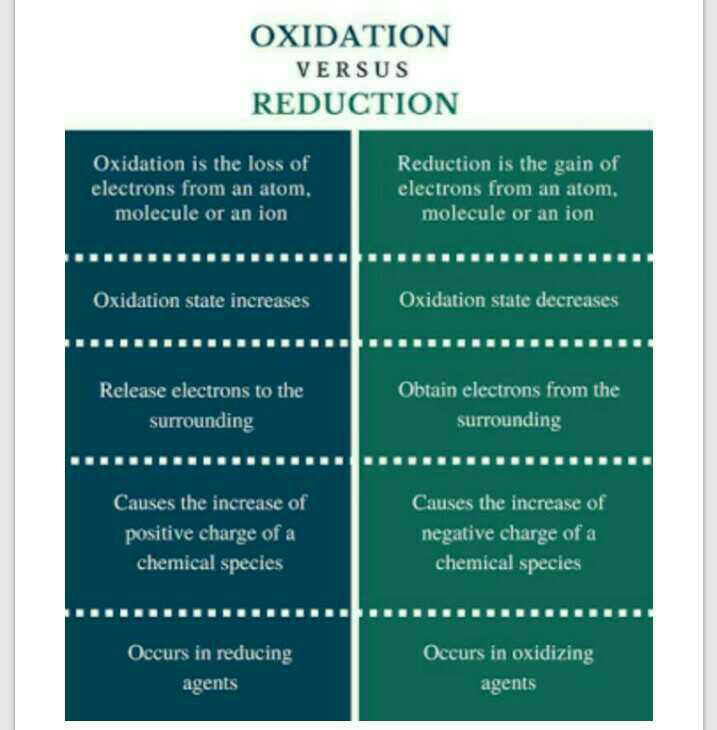 Difference Between Oxidation And Reduction Edurev Class 10
Difference Between Oxidation And Reduction Edurev Class 10
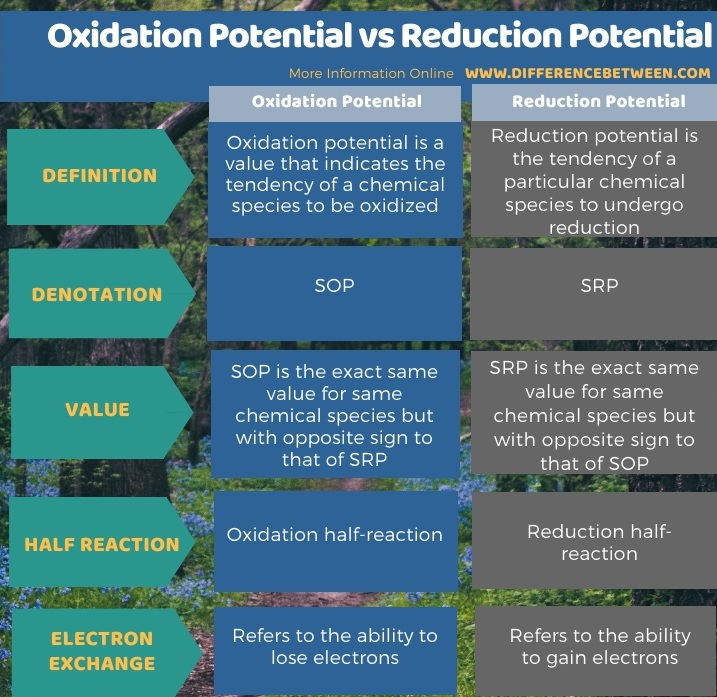 Difference Between Oxidation Potential And Reduction Potential
Difference Between Oxidation Potential And Reduction Potential
What Is The Difference Between Oxidation And Reduction Quora
 Solved Pre Lab Questions 1 What Is The Difference Betwee
Solved Pre Lab Questions 1 What Is The Difference Betwee
Difference Between Oxidation And Reduction Definition Mechanism
 What Is The Difference Between Oxidation Reduction Types Of
What Is The Difference Between Oxidation Reduction Types Of
 Difference Between Oxidation And Reduction Redox Reaction Class
Difference Between Oxidation And Reduction Redox Reaction Class
What Is The Difference Between Oxidation And Reduction Quora
 Difference Between Oxidation And Reduction Potential Brainly In
Difference Between Oxidation And Reduction Potential Brainly In
 Solved 18 1 A Briefly Explain The Difference Between Ox
Solved 18 1 A Briefly Explain The Difference Between Ox
 Redox Redox Involves Two Simultaneous Reactions An
Redox Redox Involves Two Simultaneous Reactions An
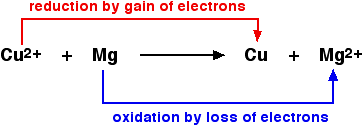 Definitions Of Oxidation And Reduction Chemistry Libretexts
Definitions Of Oxidation And Reduction Chemistry Libretexts
What Is The Difference Between Oxidation And Reduction Quora
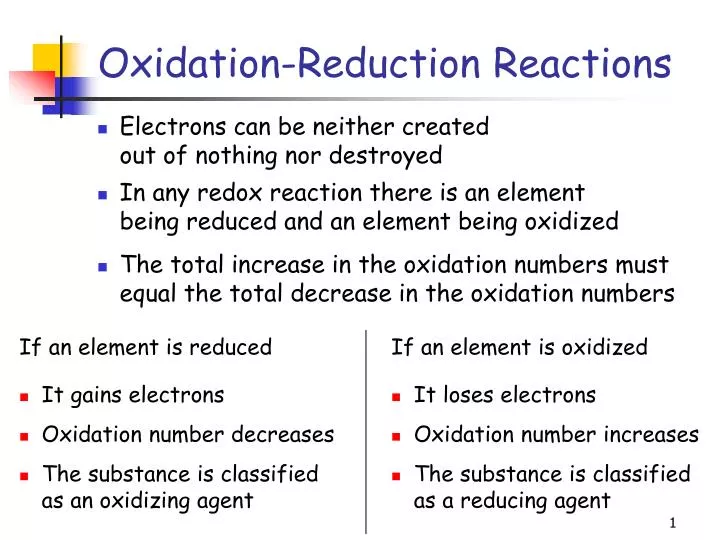 Ppt Oxidation Reduction Reactions Powerpoint Presentation Free
Ppt Oxidation Reduction Reactions Powerpoint Presentation Free
 Major Differences Difference Between Oxidation And Reduction
Major Differences Difference Between Oxidation And Reduction
 Difference Between Hydrogenation And Reduction Compare The
Difference Between Hydrogenation And Reduction Compare The
 Difference Between Oxidation And Reduction Oxidizing Agent And
Difference Between Oxidation And Reduction Oxidizing Agent And
Difference Between Oxidation Number And Oxidation State
Posting Komentar
Posting Komentar