Bicarbonate Acid Or Base
I know that the bicarbonate ion hco3 can be a base and accept a h to become carbonic acid but can it lose the h it already has and become co3 in which act like an acid. For example when working out and building muscles your muscle tissue produces lactic acid.
 Blood Acid Base Balance Responses Following Nahco 3 Or Pla Where
Blood Acid Base Balance Responses Following Nahco 3 Or Pla Where
An acid base nomogram for human plasma showing the effects on the plasma ph when carbonic acid partial pressure of carbondioxide or bicarbonate occur in excess or are deficient in the plasma.
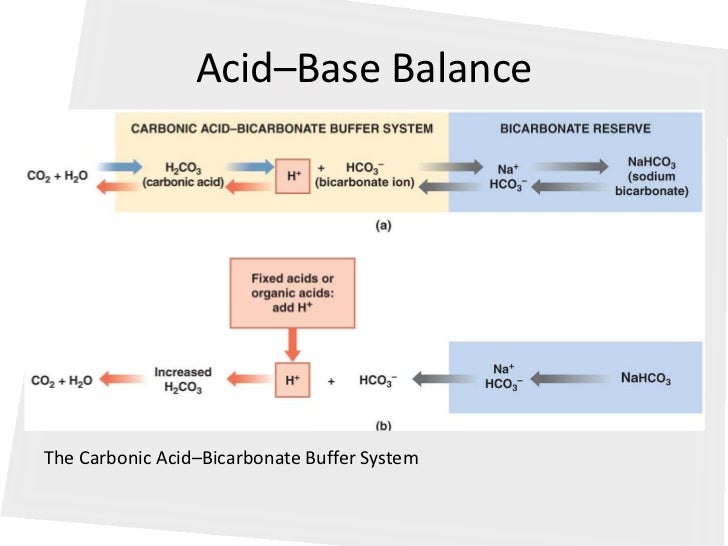
Bicarbonate acid or base. It is a conjugate base of a carbonic acid. When sodium bicarbonate nahco 3 comes into contact with a strong acid such as hcl carbonic acid h 2 co 3 which is a weak acid and nacl are formed. Bicarbonate ion is a polyatomic ion whose formula is hco3.
The prefix bi in bicarbonate comes from an outdated naming system and is based on the observation that there is twice as much carbonate per sodium ion in. It is a polyatomic anion with the chemical formula hco 3. Acid base imbalance occurs when a significant insult causes the blood ph to shift out of the normal range 7 32 to 7 42.
Because correction of the underlying metabolic disorder generally results in correction of acid base abnormalities and because of the potential risks of sodium bicarbonate therapy in the treatment of this disorder administration of sodium bicarbonate is generally reserved for the treatment of severe acidosis e g arterial ph less than 7 7 15 or serum bicarbonate concentration of 8 meq l or less. When carbonic acid comes into contact with a strong base such as naoh bicarbonate and water are formed. When carbonic acid comes into contact with a strong base such as naoh bicarbonate and water are formed.
When sodium bicarbonate nahco 3 comes into contact with a strong acid such as hcl carbonic acid h 2 co 3 which is a weak acid and nacl are formed. It has a role as a human metabolite a saccharomyces cerevisiae metabolite an escherichia coli metabolite a mouse metabolite and a cofactor. Nci thesaurus ncit hydrogencarbonate is the carbon oxoanion resulting from the removal of a proton from carbonic acid.
This acid is picked up and neutralized by the bicarbonate ion. Nahco 3 hcl h 2 co 3 nacl. In inorganic chemistry bicarbonate is an intermediate form in the deprotonation of carbonic acid.
The term bicarbonate was coined in 1814 by the english chemist william hyde wollaston. Bicarbonate serves a crucial biochemical role in the physiological ph buffering system. The wrap up acids and bases are chemical substances that release ions when dissolved in water.
 Acid Base Balence Primum Non Nocere
Acid Base Balence Primum Non Nocere
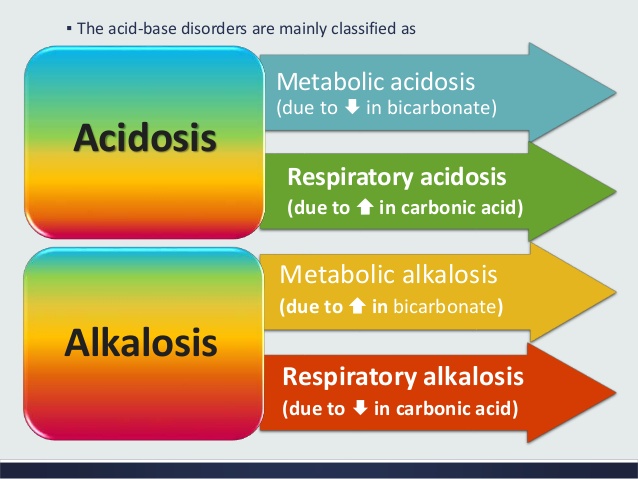 Normal Acid Base Balance And Its Disorders Medcaretips Com
Normal Acid Base Balance And Its Disorders Medcaretips Com
 Is Sodium Bicarbonate An Acid Or Alkali لم يسبق له مثيل الصور
Is Sodium Bicarbonate An Acid Or Alkali لم يسبق له مثيل الصور
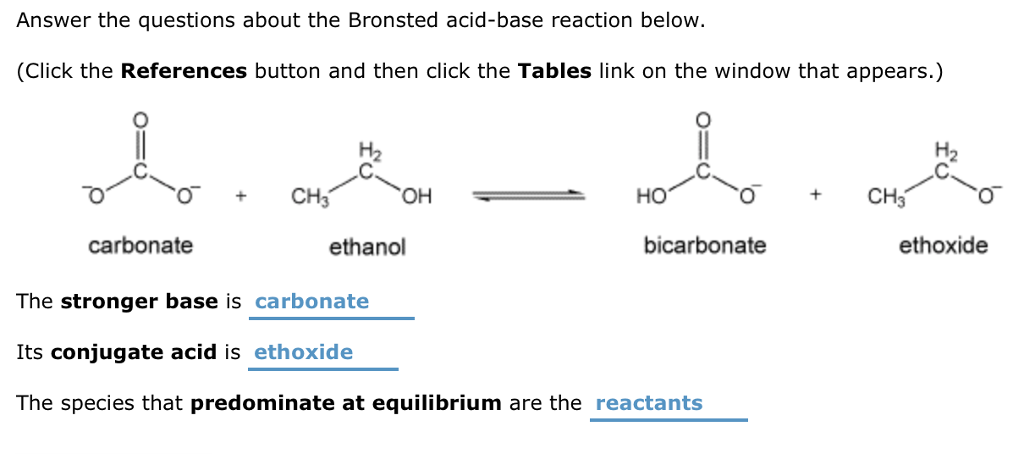 Solved Answer The Questions About The Bronsted Acid Base
Solved Answer The Questions About The Bronsted Acid Base
 Acid Base Balance Anatomy And Physiology Ii
Acid Base Balance Anatomy And Physiology Ii
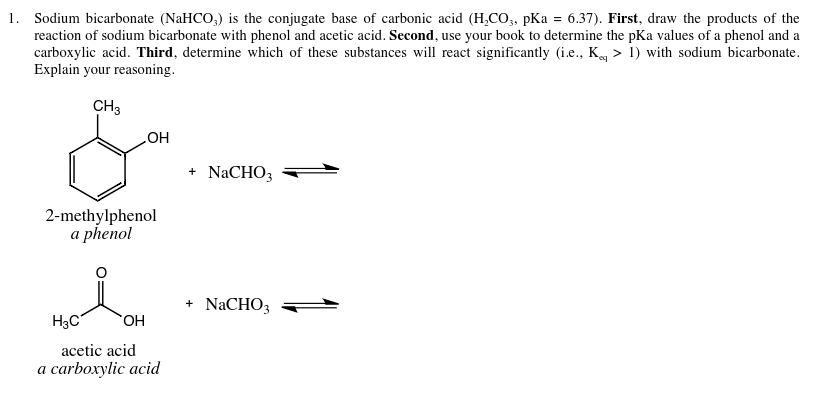 Solved Sodium Bicarbonate Nahco 3 Is The Conjugate Base
Solved Sodium Bicarbonate Nahco 3 Is The Conjugate Base
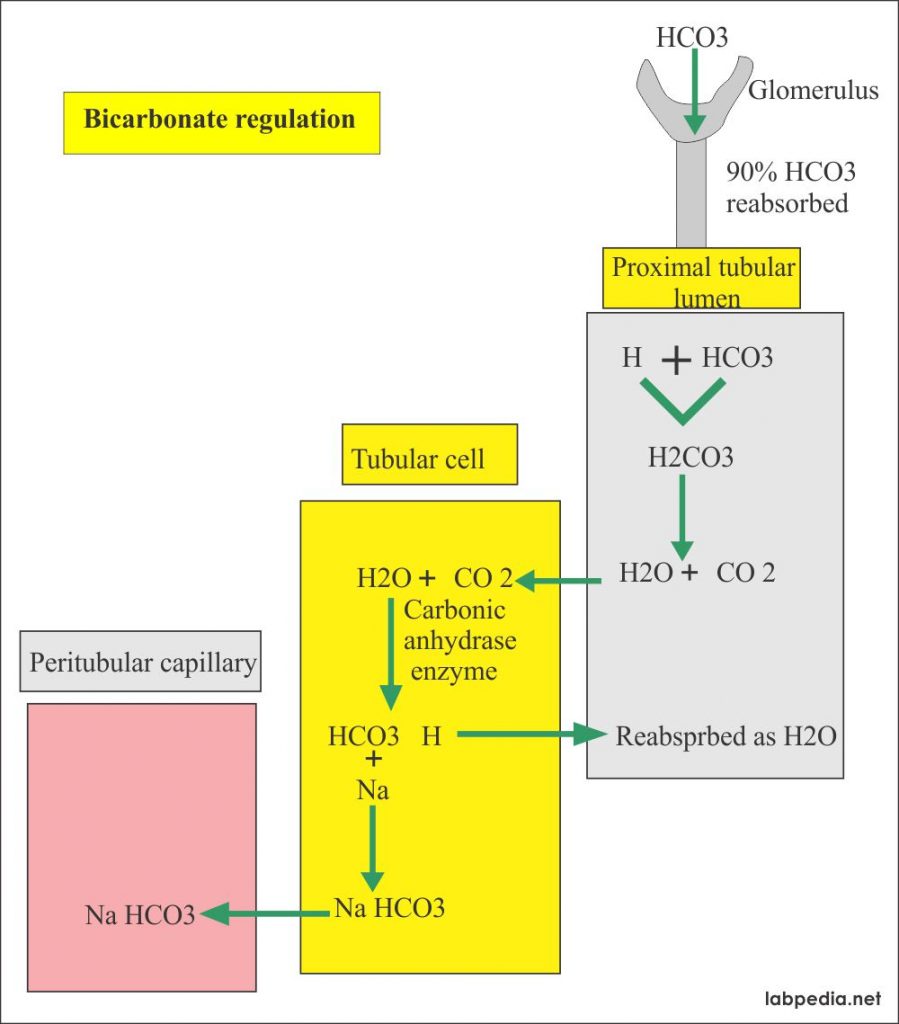 Bicarbonate Level Hco3 Acid Base Balance Labpedia Net
Bicarbonate Level Hco3 Acid Base Balance Labpedia Net
 Acids And Bases Acids Taste Sour Citric Acid Acetic Acid Bases
Acids And Bases Acids Taste Sour Citric Acid Acetic Acid Bases
 Acid Base Balance In The Kidney Team 6 P451 Fall 2011
Acid Base Balance In The Kidney Team 6 P451 Fall 2011
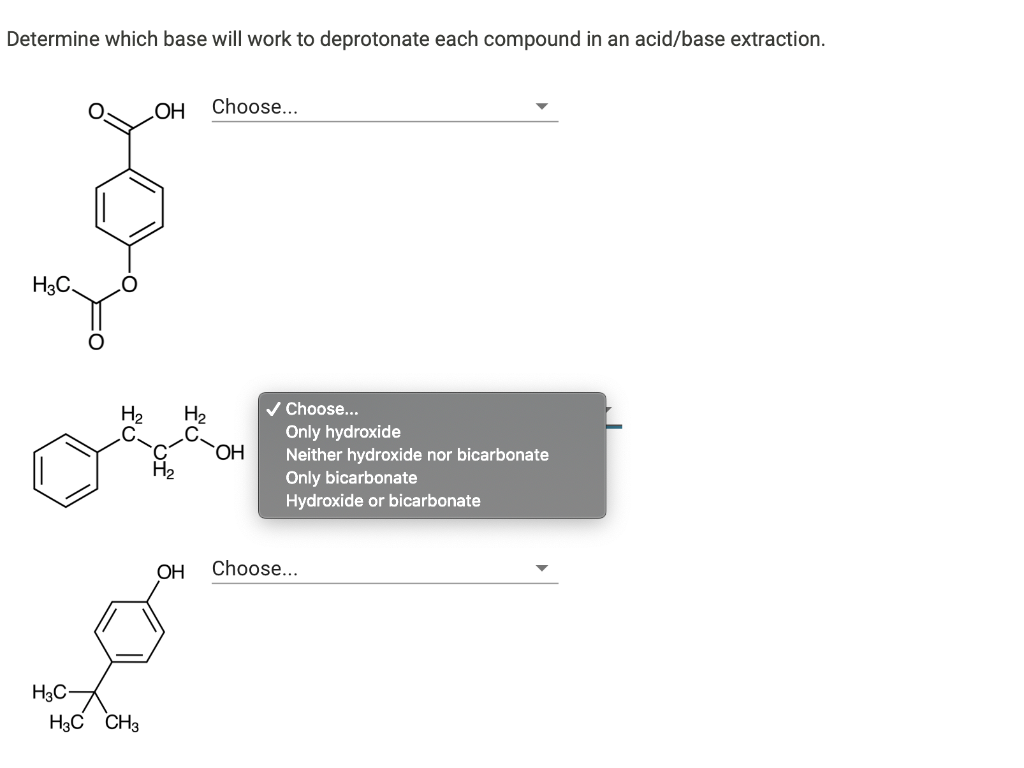 Solved Determine Which Base Will Work To Deprotonate Each
Solved Determine Which Base Will Work To Deprotonate Each
 Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctcbuqe03l5k5hvzcdic4gv4ke9rd5na4somncv8rpq5ak6i25x Usqp Cau
Acid Base Balance Understanding Is Critical To Treat Patients Jems
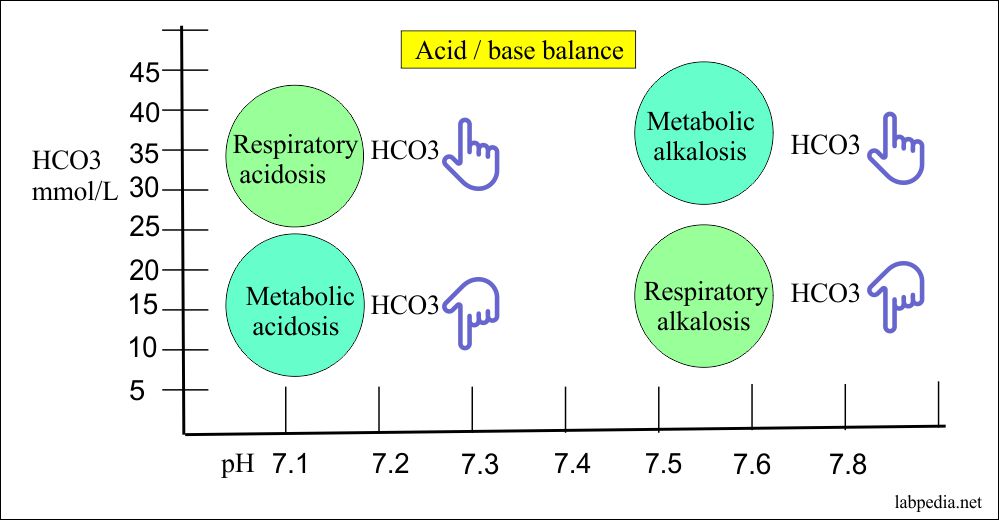 Bicarbonate Level Hco3 Acid Base Balance Labpedia Net
Bicarbonate Level Hco3 Acid Base Balance Labpedia Net
7 Acid Base Balance Functions Of Cells And Human Body
Separation Of An Unknown Mixture
 Acid Base Balance Bicarbonate Ion Buffer Youtube
Acid Base Balance Bicarbonate Ion Buffer Youtube
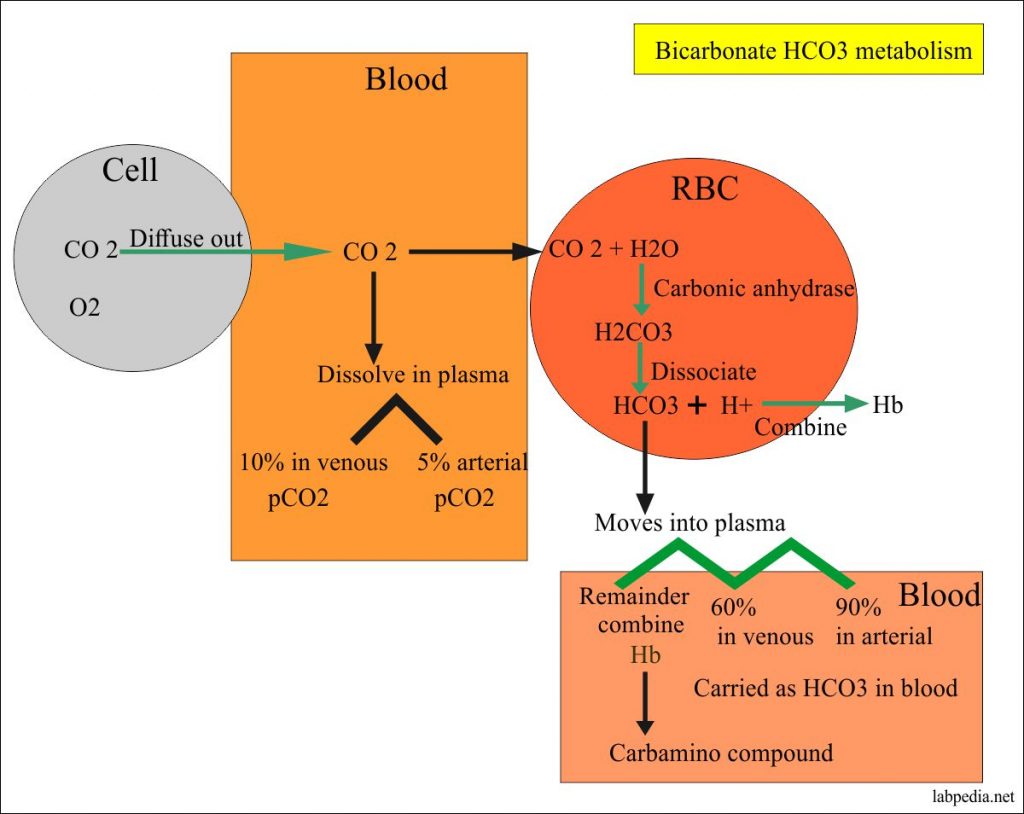 Bicarbonate Level Hco3 Acid Base Balance Labpedia Net
Bicarbonate Level Hco3 Acid Base Balance Labpedia Net
 Sodium Bicarbonate An Overview Sciencedirect Topics
Sodium Bicarbonate An Overview Sciencedirect Topics
Given The Salt Nahco3 Why Is It Basic In A Solution Quora
Separation Of An Unknown Mixture
 Acid Base Balance Renal Bicarbonate Reabsorption Abdominal Key
Acid Base Balance Renal Bicarbonate Reabsorption Abdominal Key
 Bicarbonate An Overview Sciencedirect Topics
Bicarbonate An Overview Sciencedirect Topics
Acid Base Extraction Chemistry Libretexts
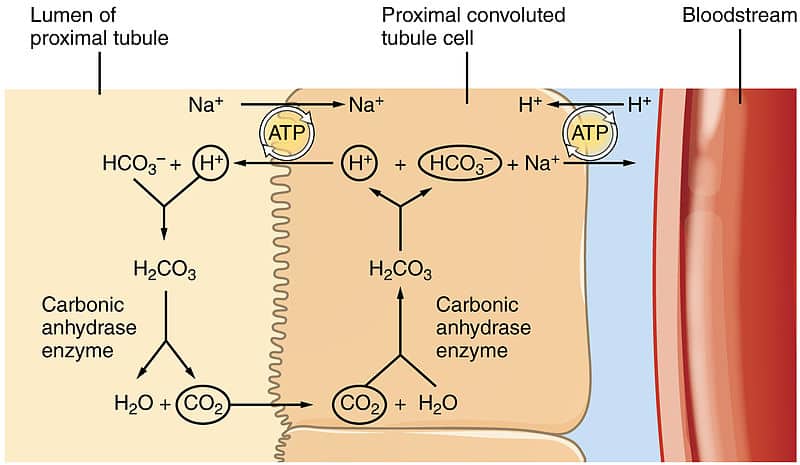 Urinary Regulation Of Acid Base Balance Hydrogen Excretion
Urinary Regulation Of Acid Base Balance Hydrogen Excretion
 Acid Base Balance Anatomy And Physiology Ii
Acid Base Balance Anatomy And Physiology Ii
 Anatomy Notes Ch 19 Diagram Quizlet
Anatomy Notes Ch 19 Diagram Quizlet
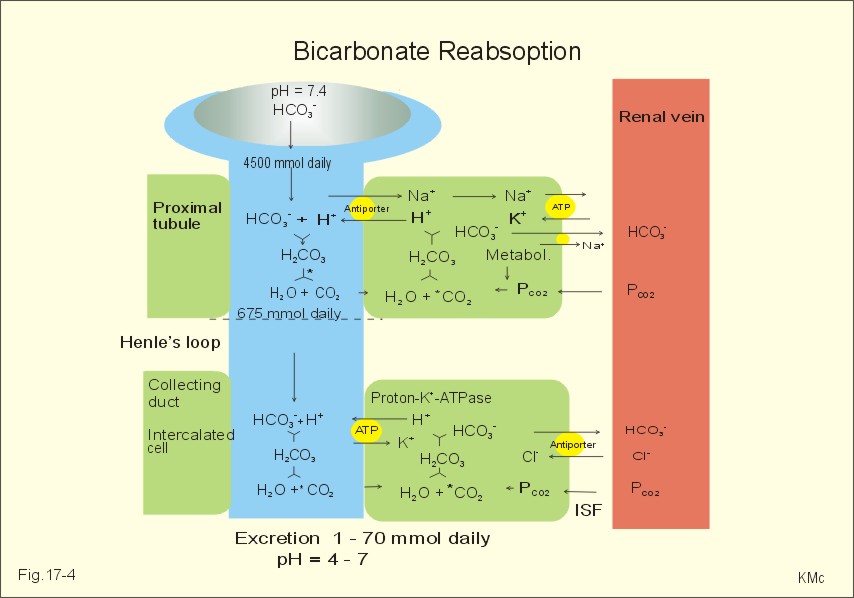

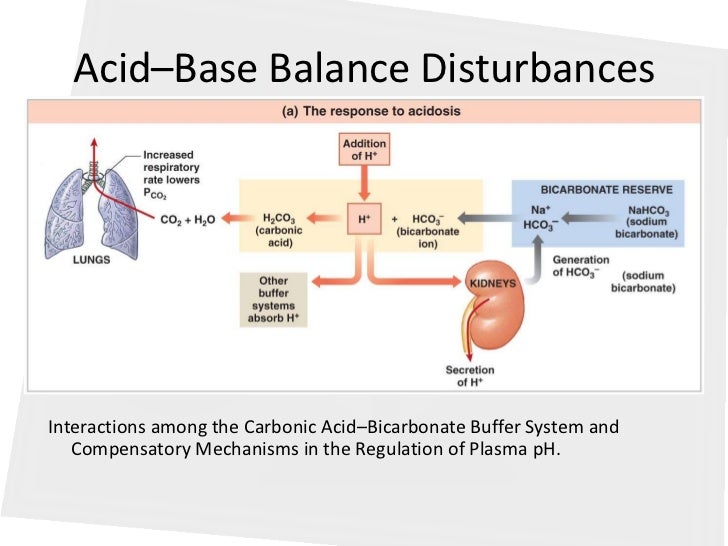
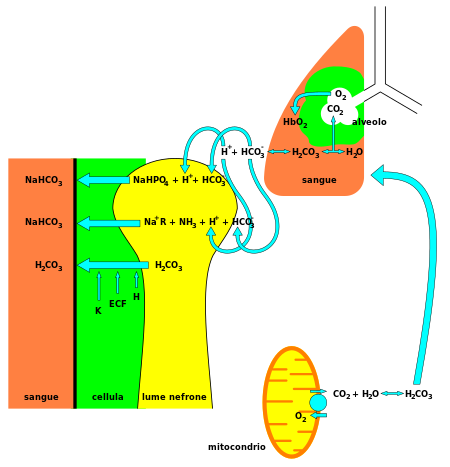

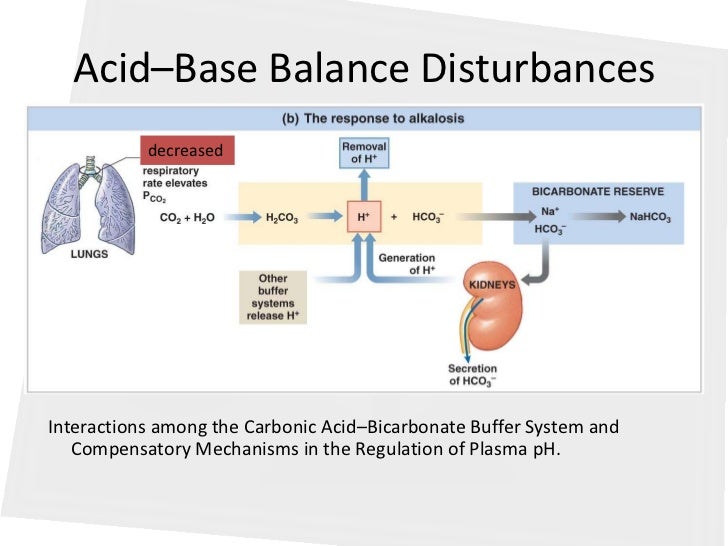
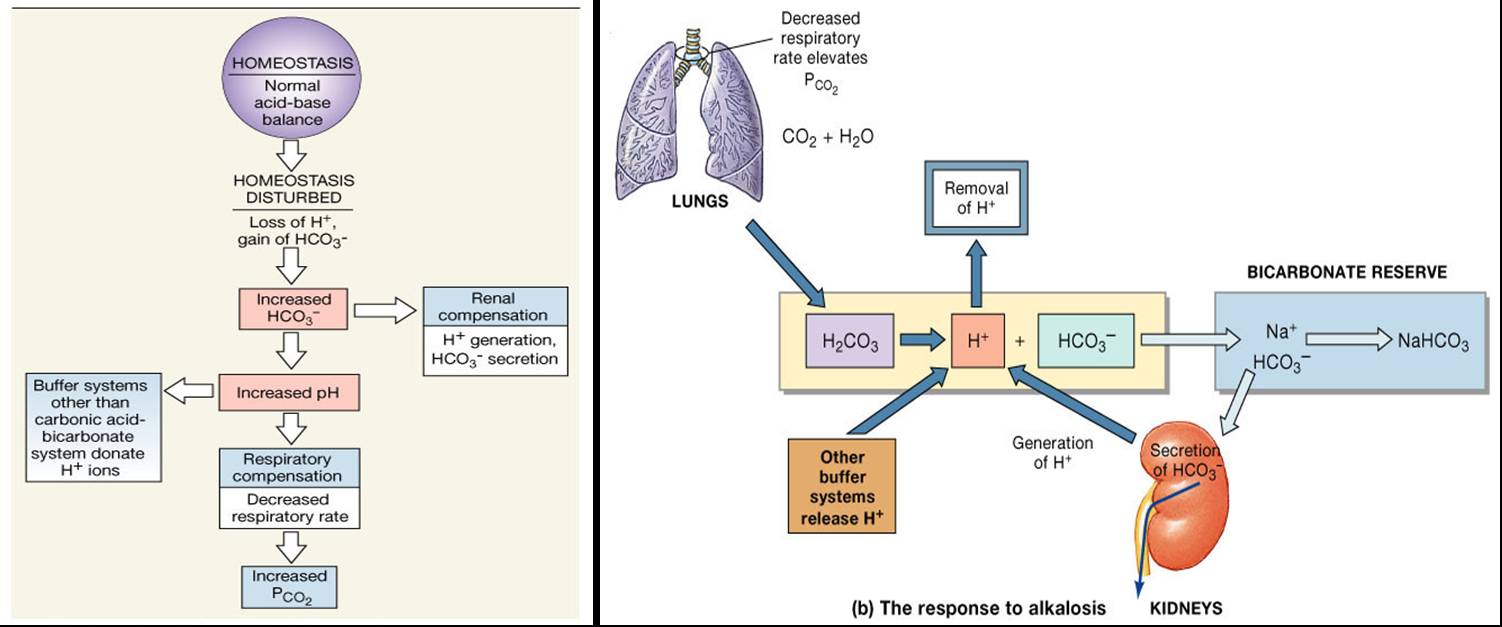

Posting Komentar
Posting Komentar