Graham S Law Of Diffusion Pdf
If the carbon dioxide in problem 1 takes 32 sec to effuse how long will the. Find the ratio of diffusion rates of hydrogen gas and oxygen gas.

In 1829 thomas graham found that at constant temperature and pressure the gas with lower molecular mass diffuses more rapidly while the gas with the higher molecular mass diffuses more slowly.

Graham s law of diffusion pdf. P u r p o s e. Graham s law of effusion. To compare the rates of diffusion of ammonia gas and hydrogen chloride gas.
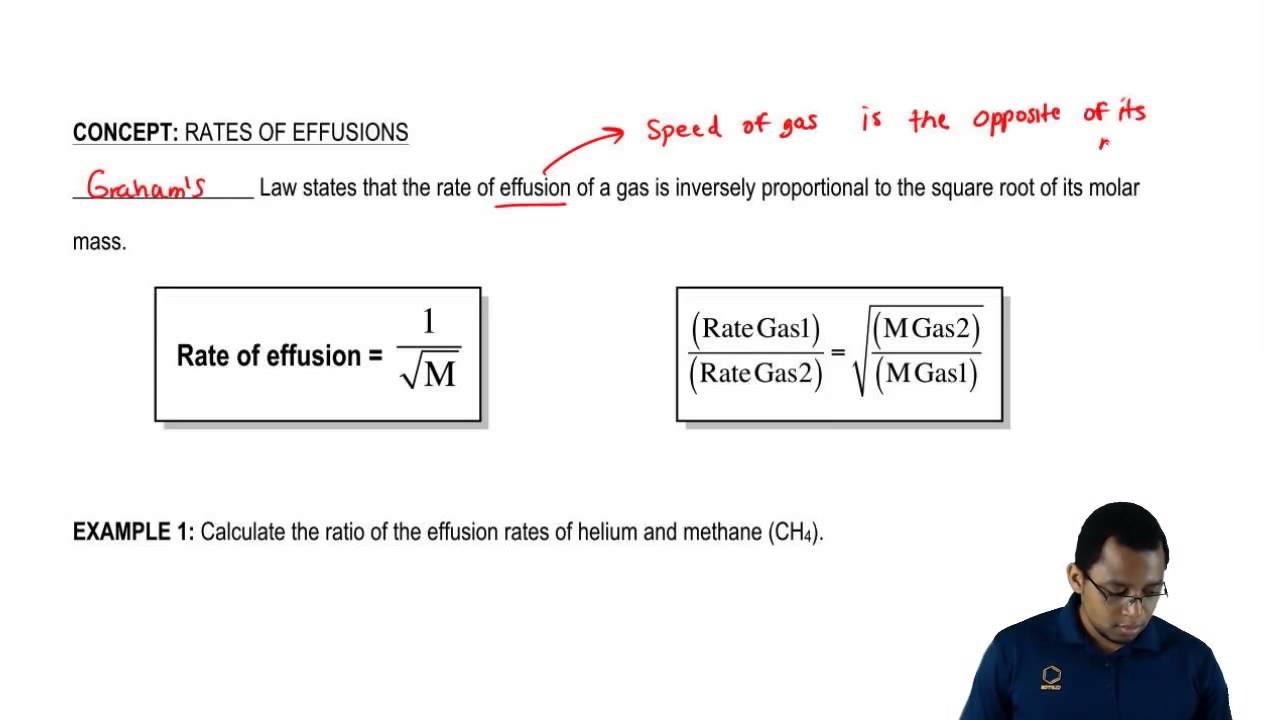
Graham s law states that the rate of effusion or diffusion of a gas is inversely proportional to the square root of the molar mass of the gas. Graham s law of diffusion effusion. Graham s law can be understood by comparing two gases ce a and ce b at the same temperature meaning the gases have the same kinetic energy.
That means the hydrogen has the speed four times greater than the speed of oxygen. Diffusion is faster at higher temperatures because the gas molecules have greater kinetic energy. Graham s law states that the effusion rate of a gas is inversely proportional to the square root of the mass of its particles.
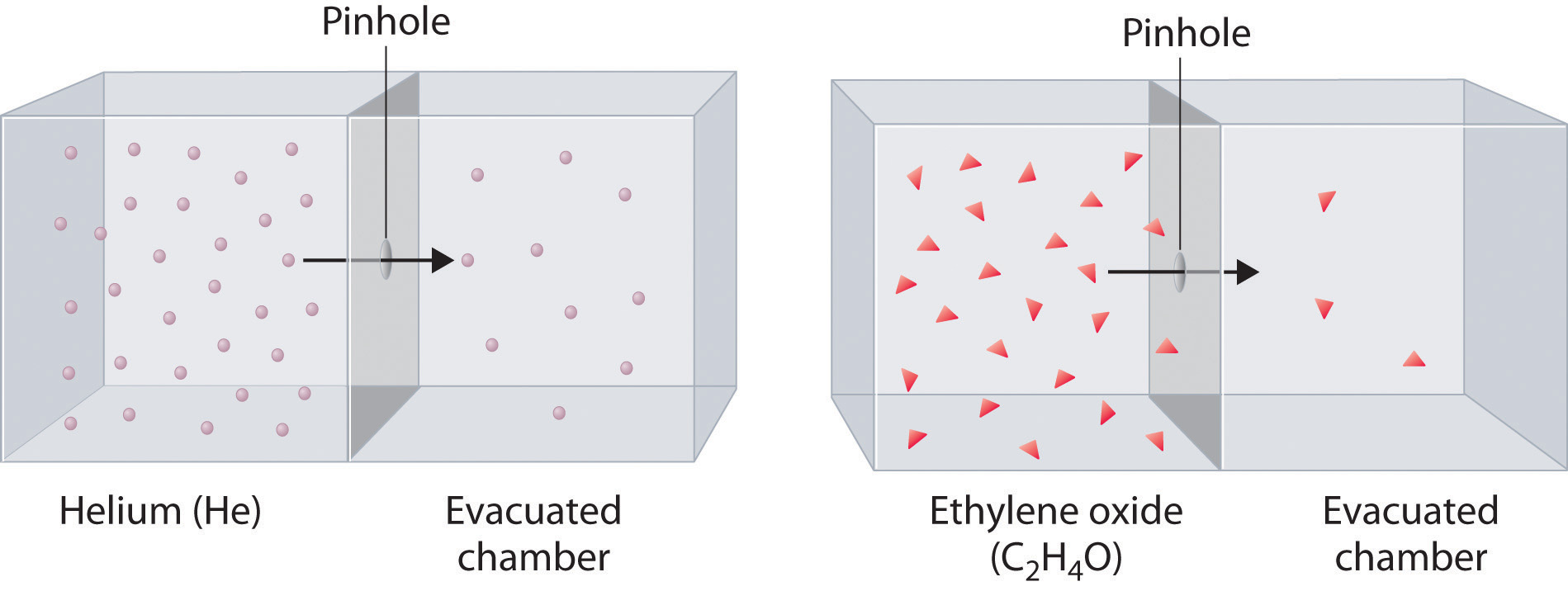
Effusion is the process that occurs when a gas is permitted to escape its container through a small opening. Problem 1 graham s law the first gas is gas a and the second gas is gas b. Diffusion in the ability of a gas to spread and occupy the whole available volume irrespective of other gases present in the container.
Effusion refers to the movement of gas particles through a small hole. In 1829 thomas graham used an apparatus similar to the one shown in figure 4 15 to study the diffusion of gases the rate at which two gases mix. Relative rate means find the ratio v a v b.
This can be interpreted as the rate of hydrogen is four times the rate of oxygen. Write down the formula for graham s law. 2 2 co h h will effuse 4 69 times faster than co 4 69 4 7 2 0g mol 44 0g mol rate rate 2 2 2.
The graham s laws for hydrogen gas and oxygen gas can be written as. Determine the relative rate of diffusion for krypton and bromine 1 381 kr diffuses 1 381 times faster than br 2. Under the same conditions of temperature and pressure how many times faster will hydrogen effuse compared to carbon dioxide.
Diffusion is the process of slowly mixing two gases together. Quantifying effusion and diffusion cont u 1 u 2 rateofeffusionforgas1 rateofeffusionforgas2 m 2 m 1 distancetraveledbygas1 distancetraveledbygas2 m 2 m 1. Effusion is the process by which a gas escapes from one chamber of a vessel through a small opening or an orifice.
In this way he slowed down the process so that it could be studied quantitatively. Graham s law of effusion. Graham s law of effusion key 1.
Kr br br kr m m v v 2 2 a b b a m m v v 83 80 g mol 159 80 g mol ex.
 Graham S Law Practice Problems Pdf Problem 1 If Equal Amounts Of
Graham S Law Practice Problems Pdf Problem 1 If Equal Amounts Of
 Grahams Law Of Effusion Sample Problem Chem In 10 Online
Grahams Law Of Effusion Sample Problem Chem In 10 Online

 According To Graham S Law At A Given Temperature The Ratio Of Th
According To Graham S Law At A Given Temperature The Ratio Of Th

 Grahams Law Of Diffusion Worksheet Pdf Fill Online Printable
Grahams Law Of Diffusion Worksheet Pdf Fill Online Printable
 Understanding Graham S Law Of Effusion Youtube
Understanding Graham S Law Of Effusion Youtube
Https Faculty Ncc Edu Linkclick Aspx Fileticket Ys50ceoa9jk 3d Tabid 1859
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcsdljcdjpgl1 Lkuhiavu Tpktv5gh5cgjje7pliwk0orlpz90p Usqp Cau
 Pdf Thomas Graham Ii Contributions To Diffusion Of Gases And
Pdf Thomas Graham Ii Contributions To Diffusion Of Gases And
 Graham S Law Of Diffusion Or Effusion Video In Hindi Youtube
Graham S Law Of Diffusion Or Effusion Video In Hindi Youtube
 2 9 Graham S Laws Of Diffusion And Effusion Chemistry Libretexts
2 9 Graham S Laws Of Diffusion And Effusion Chemistry Libretexts
 10 8 Graham S Law Science Chemistry Gas Laws Showme
10 8 Graham S Law Science Chemistry Gas Laws Showme
 Pdf Fick S Laws Of Diffusion Embe Jule Academia Edu
Pdf Fick S Laws Of Diffusion Embe Jule Academia Edu
 Physics Study Material For Class Xi Pdf By S Dharmaraj Issuu
Physics Study Material For Class Xi Pdf By S Dharmaraj Issuu

Grahams Law Of Diffusion Authorstream
 Gas Laws Graham S Law Of Effusion Practice Problems By Amy Brown
Gas Laws Graham S Law Of Effusion Practice Problems By Amy Brown
 Fillable Online Grahams Law Of Diff Worksheet App
Fillable Online Grahams Law Of Diff Worksheet App
 Grahams Law Sample Problem Youtube
Grahams Law Sample Problem Youtube
 Graham Pdf Graham U2019s Law Of Effusion Key 1 Under The Same
Graham Pdf Graham U2019s Law Of Effusion Key 1 Under The Same
 Pdf Graham S Law Of Diffusion Quantum Analogy And Non Ideality
Pdf Graham S Law Of Diffusion Quantum Analogy And Non Ideality
Http Www Celinaschools Org Downloads Grahams 20law 20of 20effusion Pdf
 Graham S Law Of Effusion Worksheet Grahams Law Or Effusion Nome
Graham S Law Of Effusion Worksheet Grahams Law Or Effusion Nome
 Pdf Graham S Law Of Diffusion Quantum Analogy And Non Ideality
Pdf Graham S Law Of Diffusion Quantum Analogy And Non Ideality
 Effusion And Diffusion Of Gases Chemistry Atoms First
Effusion And Diffusion Of Gases Chemistry Atoms First
 The Graham S Law States That At Constant Pressure And Temperatur
The Graham S Law States That At Constant Pressure And Temperatur
 Gas Laws Graham S Law Of Effusion Practice Problems By Amy Brown
Gas Laws Graham S Law Of Effusion Practice Problems By Amy Brown
 Chemistry Gas Laws Graham S Law Of Diffusion By William Hicks Tpt
Chemistry Gas Laws Graham S Law Of Diffusion By William Hicks Tpt
 Chemistry Notes Chemistry Pdf Gases Gas Laws And Ideal Gas Law
Chemistry Notes Chemistry Pdf Gases Gas Laws And Ideal Gas Law
 Graham S Law Of Effusion Worksheet Grahams Law Or Effusion Nome
Graham S Law Of Effusion Worksheet Grahams Law Or Effusion Nome
 The Graham S Law States That At Constant Pressure And Temperatur
The Graham S Law States That At Constant Pressure And Temperatur
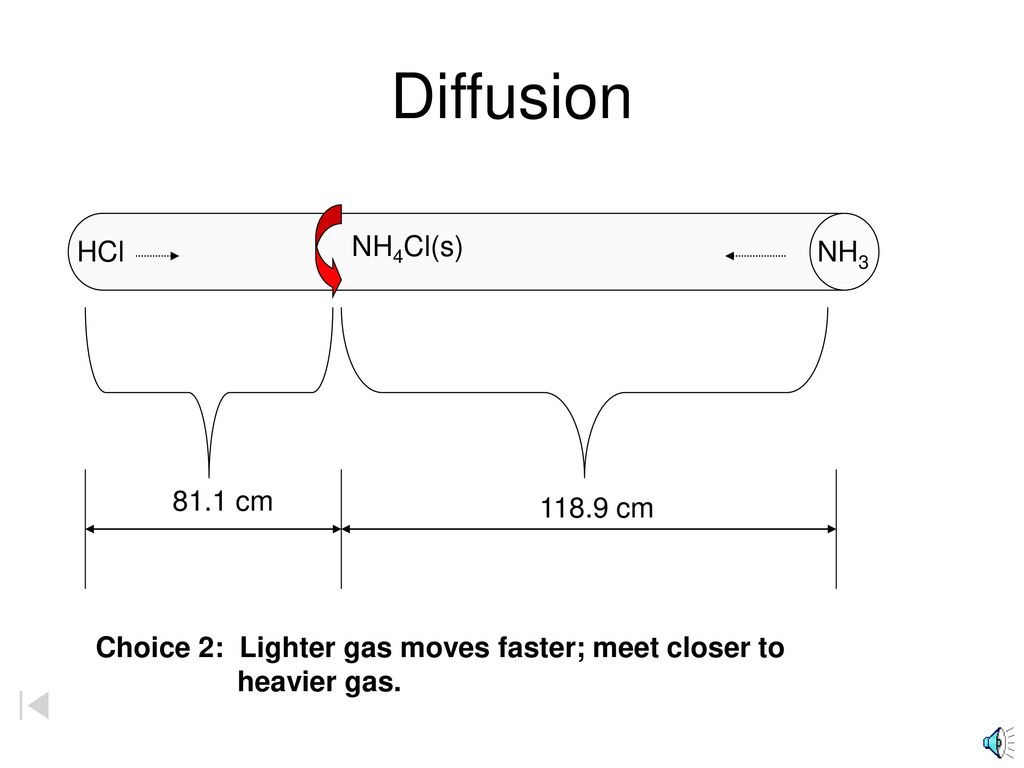 Graham S Law Of Diffusion Ppt Download
Graham S Law Of Diffusion Ppt Download
 Graham S Law Of Diffusion Youtube
Graham S Law Of Diffusion Youtube

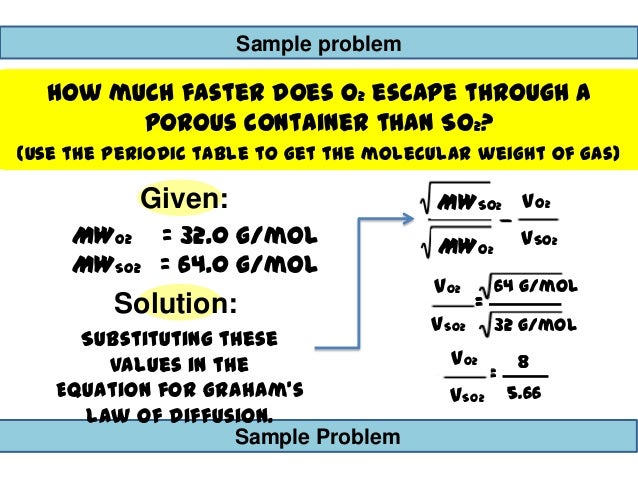
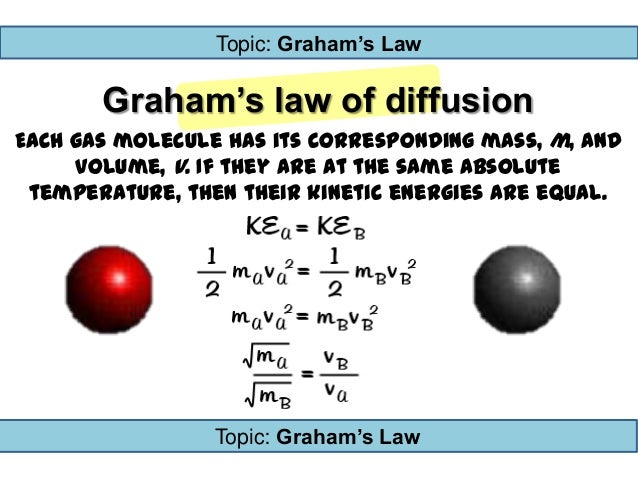


Posting Komentar
Posting Komentar