How To Tell If A Reaction Is Spontaneous Or Not
A spontaneous reaction is one in which product formation is favored even if the reaction is extremely slow. So hopefully this helps you understand if a reaction is spontaneous or not using gibbs free energy.
Difference Between Spontaneous And Nonspontaneous Reactions
If e o for the redox reaction as written is negative the reaction does not proceed in the forward direction and is said to be non spontaneous.
How to tell if a reaction is spontaneous or not. You do not have to worry about a piece of paper on your desk suddenly bursting into flames although its combustion is a spontaneous reaction. Also just a side note when delta g equal 0 which sometimes will we know that the reaction is equilibrium. I assume that your level of chemistry is already good enough for you not needing a definition for gibbs free energy hence i will go immediately into the calculations.
To be able to tell if a reaction is spontaneous or not qualitatively meaning without having to calculate anything the first thing we look at is the entropy. G h t s this is the most common way to approach this g gibbs free energy. What is missing is the required activation energy to get the reaction started.
There are ways to tell if the entropy. You will need to refer to a table of standard reduction potentials in order to determine the standard electrode potential for a given redox reaction. A spontaneous reaction may involve an increase or decrease in enthalpy it may involve an increase or decrease in entropy but it will always involve a decrease in free energy that is a negative δg.
And what that means is the forward reaction happens as well the backward reaction both occur. A reaction s spontaneity i e. If it occurs can be deduced from the gibbs free energy values.
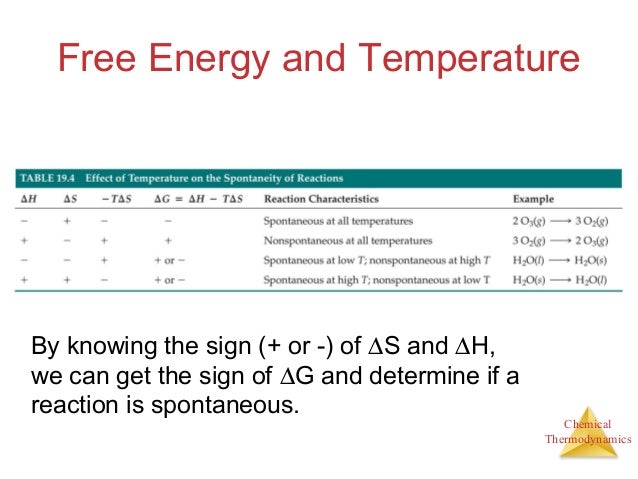
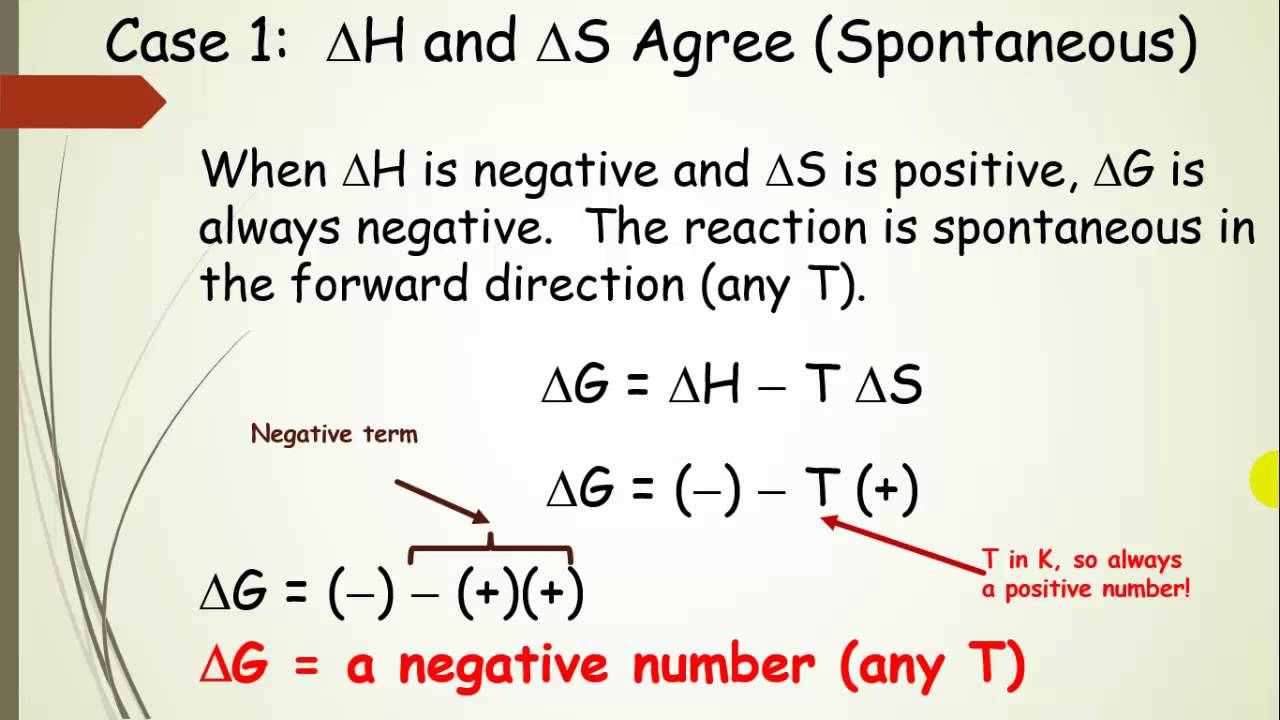 Free Energy And Predicting Spontaneous Reactions With H And S Pt
Free Energy And Predicting Spontaneous Reactions With H And S Pt
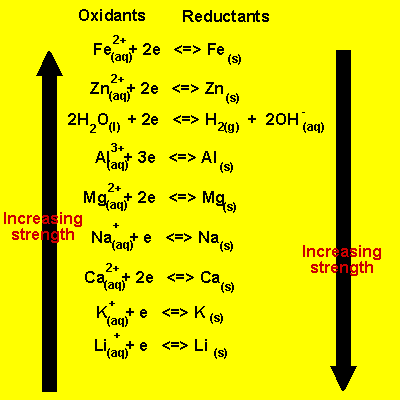 Standard Reduction Potentials Boundless Chemistry
Standard Reduction Potentials Boundless Chemistry

 Energetics Ib Topics 5 15 Part 4 Entropy Spontaneity Ppt
Energetics Ib Topics 5 15 Part 4 Entropy Spontaneity Ppt
 Identifying Spontaneous Redox Reactions Chemistry Stack Exchange
Identifying Spontaneous Redox Reactions Chemistry Stack Exchange
 Mnemonic To Remember Conditionals Upon When A Reaction Is
Mnemonic To Remember Conditionals Upon When A Reaction Is
 Gibbs Free Energy And Spontaneity Article Khan Academy
Gibbs Free Energy And Spontaneity Article Khan Academy
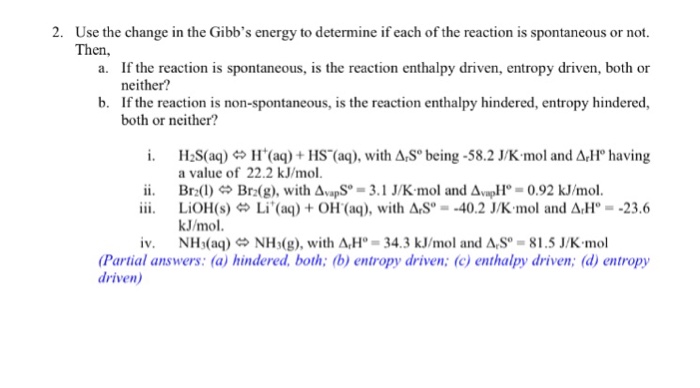
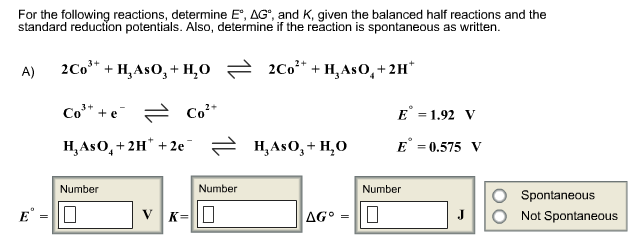 Solved For The Following Reactions Determine E Degree A
Solved For The Following Reactions Determine E Degree A
 Spontaneous Reaction Definition Examples Video Lesson
Spontaneous Reaction Definition Examples Video Lesson
 Spontaneity And Redox Reactions Video Khan Academy
Spontaneity And Redox Reactions Video Khan Academy
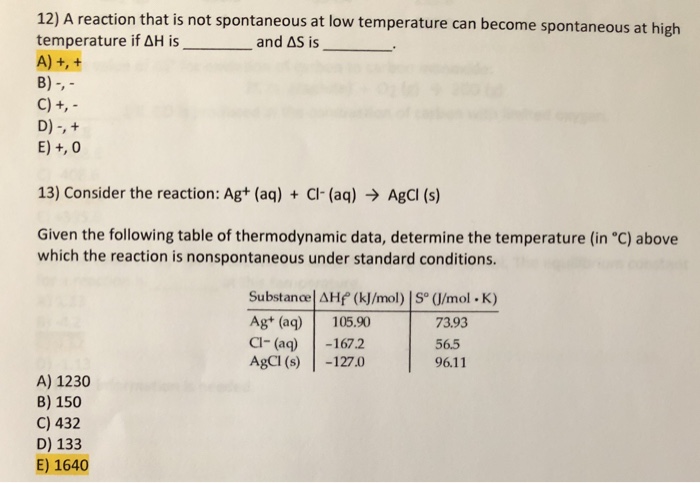
 Reaction Spontaneity 1 Spontaneous Process First Law Of
Reaction Spontaneity 1 Spontaneous Process First Law Of
Http Www2 Onu Edu S Bates Chem172 Ch18presstudent Pdf
 Spontaneous Reactions Are Reactions That Once Started Continues
Spontaneous Reactions Are Reactions That Once Started Continues
 New Chm 152 Unit 6 Power Points Sp13
New Chm 152 Unit 6 Power Points Sp13
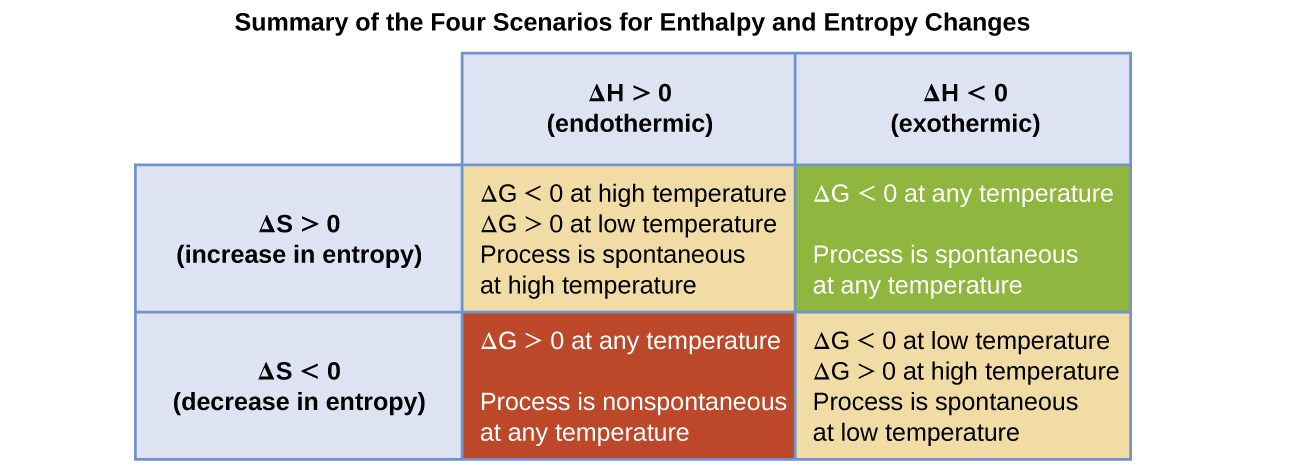
 Aleks Using The Conditions Of Spontaneity To Deduce The Signs Of
Aleks Using The Conditions Of Spontaneity To Deduce The Signs Of
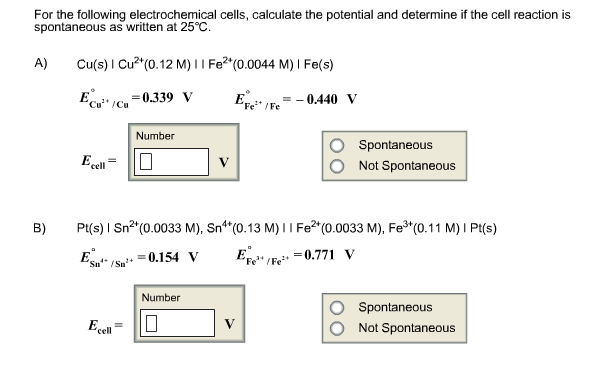 Solved For The Following Electrochemical Cells Calculate
Solved For The Following Electrochemical Cells Calculate
 Determine If Redox Reactions Are Spontaneous Under Standard
Determine If Redox Reactions Are Spontaneous Under Standard
 Free Energy Endergonic Vs Exergonic Reactions Article Khan
Free Energy Endergonic Vs Exergonic Reactions Article Khan
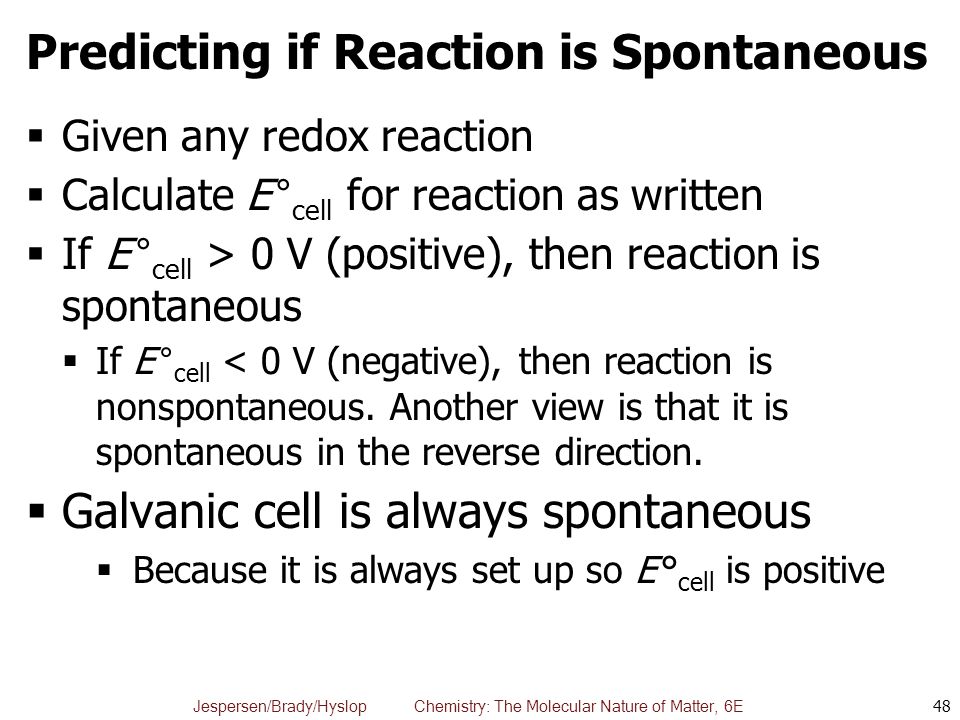 Chapter 20 Electrochemistry Ppt Download
Chapter 20 Electrochemistry Ppt Download
 Gibbs Free Energy And Spontaneous Reactions Video Khan Academy
Gibbs Free Energy And Spontaneous Reactions Video Khan Academy
 Spontaneous Reaction Definition Examples Video Lesson
Spontaneous Reaction Definition Examples Video Lesson
 Spontaneous Reaction Definition Examples Video Lesson
Spontaneous Reaction Definition Examples Video Lesson
 Is Every Endo Thermic Reaction A Non Spontaneous Reaction Quora
Is Every Endo Thermic Reaction A Non Spontaneous Reaction Quora
 Solved Determine Whether Or Not Each Redox Reaction Occur
Solved Determine Whether Or Not Each Redox Reaction Occur
/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png) Endergonic Vs Exergonic Reactions And Processes
Endergonic Vs Exergonic Reactions And Processes
 19 1 Predict If A Reaction Will Be Spontaneous Using Standard
19 1 Predict If A Reaction Will Be Spontaneous Using Standard
 Entropy And Free Energy Learning Objectives Use The Gibbs Free
Entropy And Free Energy Learning Objectives Use The Gibbs Free
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctcgtijo Vzbctxtl1 Igwdfowo F65 Kgkbdbw Vyvt9olaj14 Usqp Cau
Spontaneous Reaction Definition Examples Video Lesson
 Is It A Spontaneous Reaction Delta G Tells You Youtube
Is It A Spontaneous Reaction Delta G Tells You Youtube
 How To Identify If A Redox Reaction Is Spontaneous Or
How To Identify If A Redox Reaction Is Spontaneous Or
 Spontaneous Reactions In The Context Of A Chemical Reaction
Spontaneous Reactions In The Context Of A Chemical Reaction
 Above What Temperature Does The Following Reaction Be
Above What Temperature Does The Following Reaction Be
Https Ww2 Odu Edu Ppleban Pdf Zumdahl16 Pdf
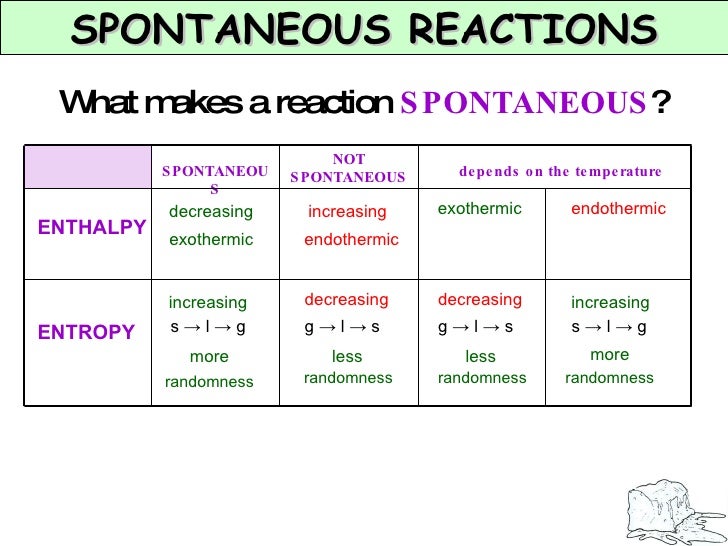 Chapter 7 Kinetics And Equilibrium
Chapter 7 Kinetics And Equilibrium
Posting Komentar
Posting Komentar