Molar Heat Capacity Formula
The heat of combustion of 1 gram of ethanol equals 29 782 j or 29 780 kj. Indeed it is the heat capacity of the object that consists of an avogadro number of molecules of the substance.
 Molar Heat Capacity Of Lead Calculation Youtube
Molar Heat Capacity Of Lead Calculation Youtube
Molar heat capacity is specific heat capacity per unit mass.

Molar heat capacity formula. 1 g of ethanol is equal to 0 0217 moles. The molar heat capacity formula is when you multiply the specific heat by molar mass. The molar heat capacity of a substance has the same dimension as the heat capacity of an object.
Specific heat capacity in chemistry. The molar specific heat capacity of a substance is nothing but the amount of heat you need to provide to raise the temperature of one gram molecule of the substance through one degree centigrade. Q heat capacity j.
This equation is written as. The greater the heat capacity the more heat is required to raise the temperature. This is the specific heat of the object a defined physical chemical property multiplied by its mass and the change in temperature.
To find the number of moles divide the quantity of the sample by its molar mass. The si units for molar heat capacity are joules mole kelvin. Q mc δ t.
So to raise the temperature of µ moles of solid through t you would need an amount of heat equal to q µ c t. In statistical mechanics entropy is an extensive property of a thermodynamic system. The si unit of molar heat capacity is the joule so molar heat capacity is expressed in terms of j mol k.
Typically molar heat of combustion is given as kj mol so 29 78 0 0217 1 370. Heat capacity mass x specific heat x change in temperature. The equation relating the heat energy q to molar heat and a temperature change work in a slightly different way than those for specific heat capacity.
M mass g. It quantifies the number ω of microscopic configurations known as microstates that are con. If you scroll back up and take a look at the formula for molar heat capacity c m c n you will find the term n which represents the number of moles of the sample.
C specific heat of object j g ºc δt change in temperature ºc. The molar heat capacity is the amount of heat that must be added to raise the temperature of 1 mol of a substance by 1 degree. Namely l 2 m t 2 θ 1 or m l t 2 θ.
Molar heat capacity is the amount of heat needed to raise the temperature of 1 mole of a substance by 1 kelvin.
 Molar Heat Capacity Hindi Thermal Physics Iit Jee Unacademy
Molar Heat Capacity Hindi Thermal Physics Iit Jee Unacademy
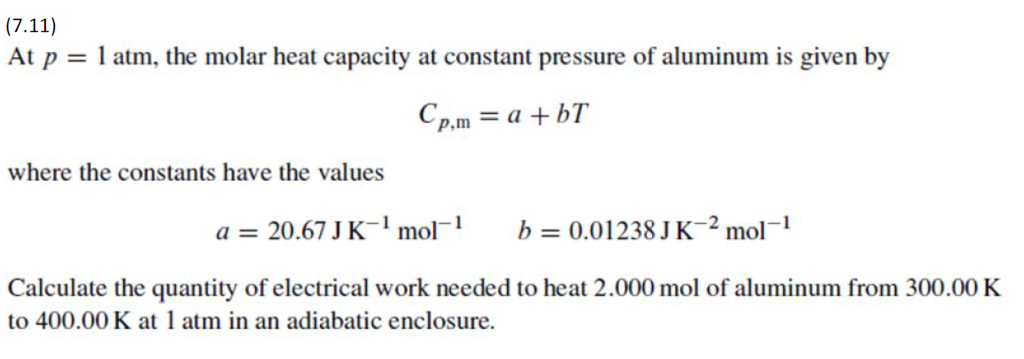 Solved At P 1 Atm The Molar Heat Capacity At Constant
Solved At P 1 Atm The Molar Heat Capacity At Constant
 Derivation Of Heat Capacity At Constant Pressure And Temperature
Derivation Of Heat Capacity At Constant Pressure And Temperature
 Answer The Molar Heat Capacity Of Silver Clutch Prep
Answer The Molar Heat Capacity Of Silver Clutch Prep
 Mean Molar Heat Capacities Of Isopropyl Alcohol Between 423 K And
Mean Molar Heat Capacities Of Isopropyl Alcohol Between 423 K And
 Thermochemistry Ppt Video Online Download
Thermochemistry Ppt Video Online Download
 Molar Heat Capacity Problems Physics Youtube
Molar Heat Capacity Problems Physics Youtube
 Heat Capacity And Specific Heat Chemistry Tutorial Youtube
Heat Capacity And Specific Heat Chemistry Tutorial Youtube
 7 2d Calculating Molar Heat Capacity Youtube
7 2d Calculating Molar Heat Capacity Youtube
 Molar Heat Capacity Sample Problems
Molar Heat Capacity Sample Problems
 For An Ideal Monoatomic Gas The Universal Gas Constant R Is N Tim
For An Ideal Monoatomic Gas The Universal Gas Constant R Is N Tim
 Molar Heat Capacity Definition Formula Equation Calculation
Molar Heat Capacity Definition Formula Equation Calculation
 Heat Capacity At Constant Volume And Pressure Video Khan Academy
Heat Capacity At Constant Volume And Pressure Video Khan Academy
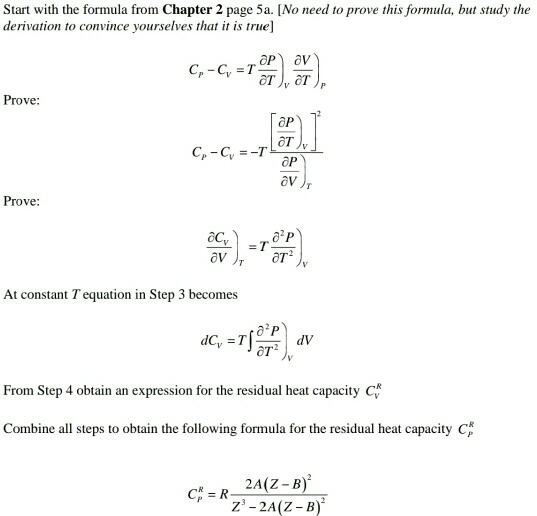 Problem 2 Calculation Of Liquid Heat Capacity Und Chegg Com
Problem 2 Calculation Of Liquid Heat Capacity Und Chegg Com
 Lecture 14 Maxwell Boltzmann Distribution Heat Capacities
Lecture 14 Maxwell Boltzmann Distribution Heat Capacities
 Calculate Average Molar Heat Capacity At Constant Toppr Com
Calculate Average Molar Heat Capacity At Constant Toppr Com
Find The Molar Heat Capacity Of An Ideal Gas In A Polytropic
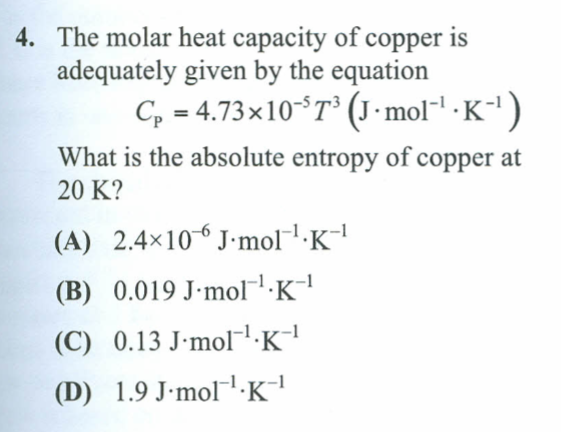 Solved The Molar Heat Capacity Of Copper Is Adequately Gi
Solved The Molar Heat Capacity Of Copper Is Adequately Gi
 Happy Mole Day Mole Day Is Celebrated Every October 23 Rd
Happy Mole Day Mole Day Is Celebrated Every October 23 Rd
 Internal Energy Of An Ideal Gas Molar Heat Capacity Of Monatomic
Internal Energy Of An Ideal Gas Molar Heat Capacity Of Monatomic
What Is Molar Heat Capacity Derive The Expression For It Physics
 Find The Molar Heat Capacity In Terms Of R Of A Monoatomic
Find The Molar Heat Capacity In Terms Of R Of A Monoatomic
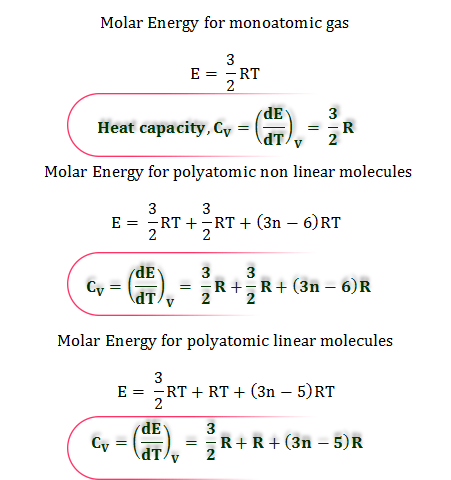 Heat Capacity Of Gases Specific Molar Priyamstudycentre
Heat Capacity Of Gases Specific Molar Priyamstudycentre
 Pdf Heat Capacities And Thermodynamic Properties Of Braunite
Pdf Heat Capacities And Thermodynamic Properties Of Braunite
What Is The Cgs Unit Of Specific Heat Capacity Quora
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcr2unkfdmpdgz5cbfoun0dwxmwnvjyx9ebpr92agkvq42veeidy Usqp Cau
Telecharger Heat Capacity Pdf Specific Formula Equation Molar
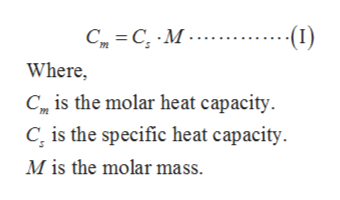 Answered The Specific Heat Of Aluminum Is 0 900 Bartleby
Answered The Specific Heat Of Aluminum Is 0 900 Bartleby
 Shows Standard Enthalpy Of Formation Standard Entropy Standard
Shows Standard Enthalpy Of Formation Standard Entropy Standard

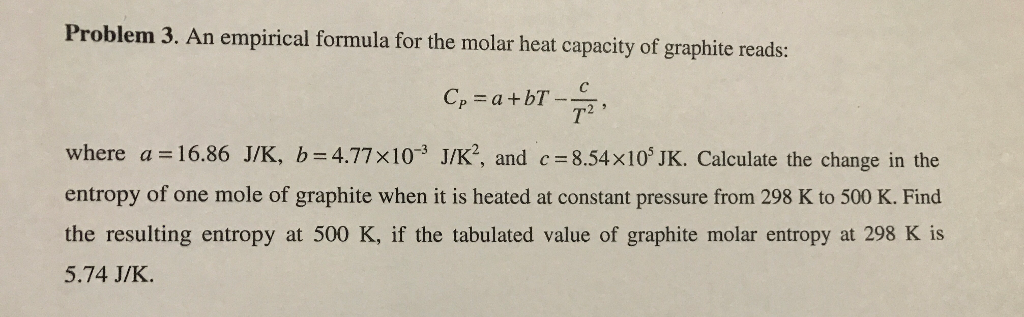 Solved Problem 3 An Empirical Formula For The Molar Heat
Solved Problem 3 An Empirical Formula For The Molar Heat
Thermal Conductivity And The Wiedemann Franz Law
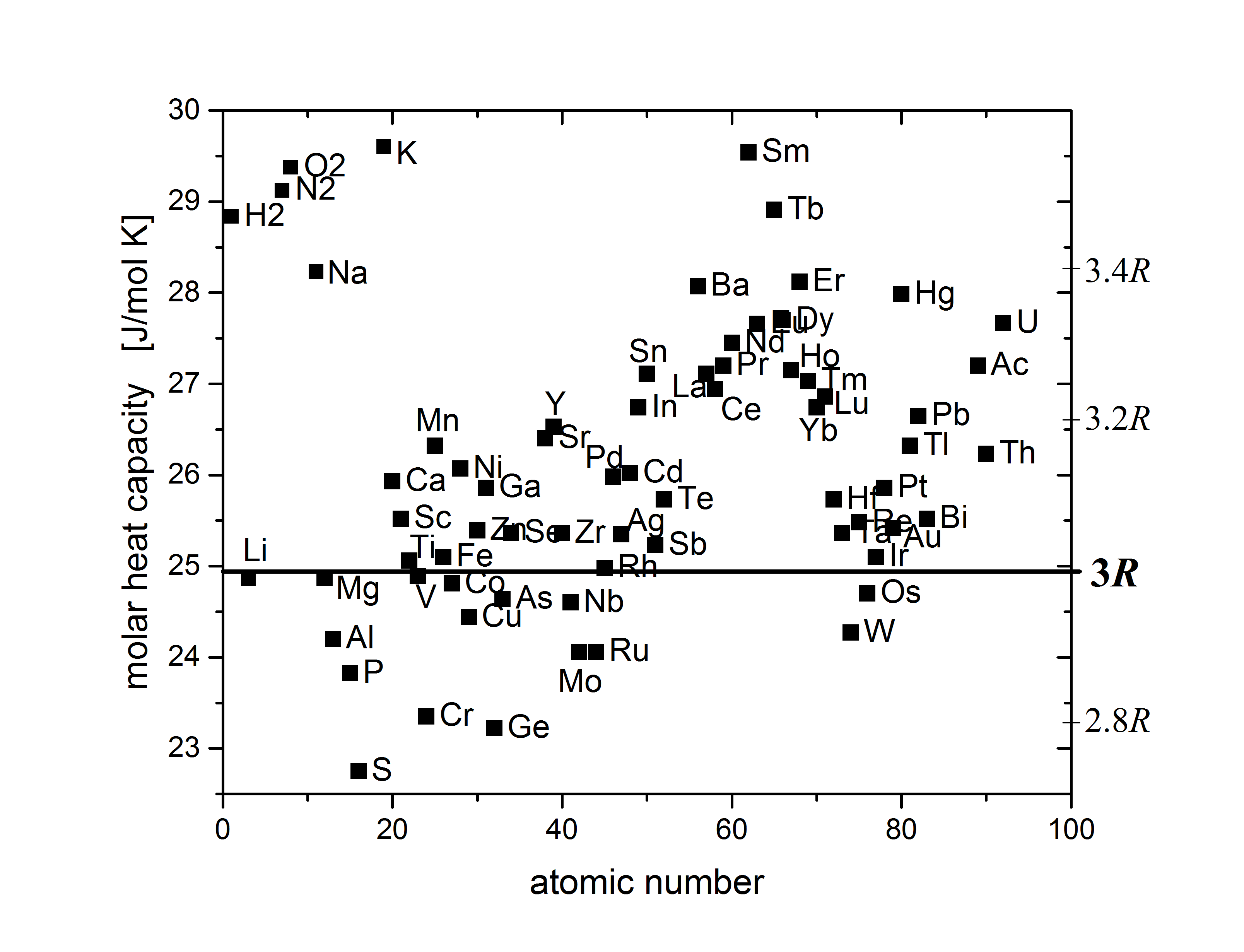



Posting Komentar
Posting Komentar