Maximum Number Of Electrons In A Subshell Formula
Each g subshell holds at most 18 electrons. Of elements 12.
 Rule To Determine The Total Number Of Elements In A Period
Rule To Determine The Total Number Of Elements In A Period
An electron shell is a group of atomic orbitals with a principal quantum number n of the same value.

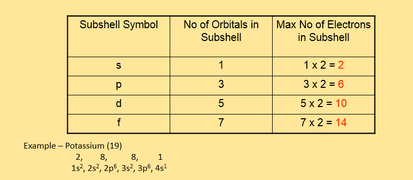
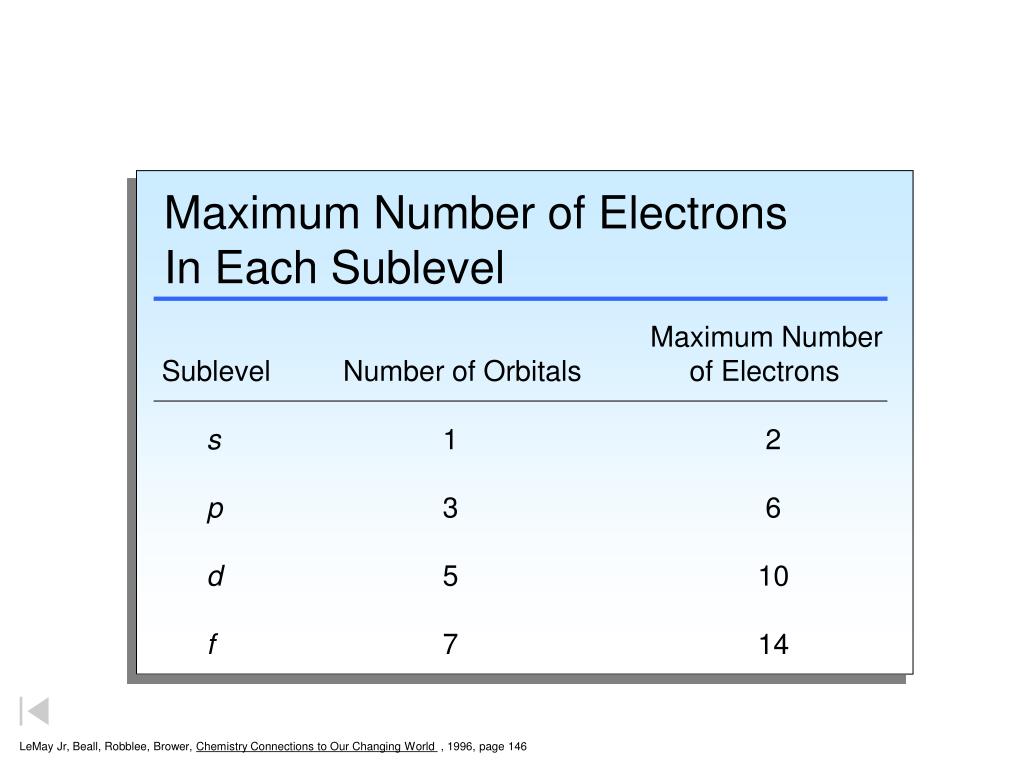
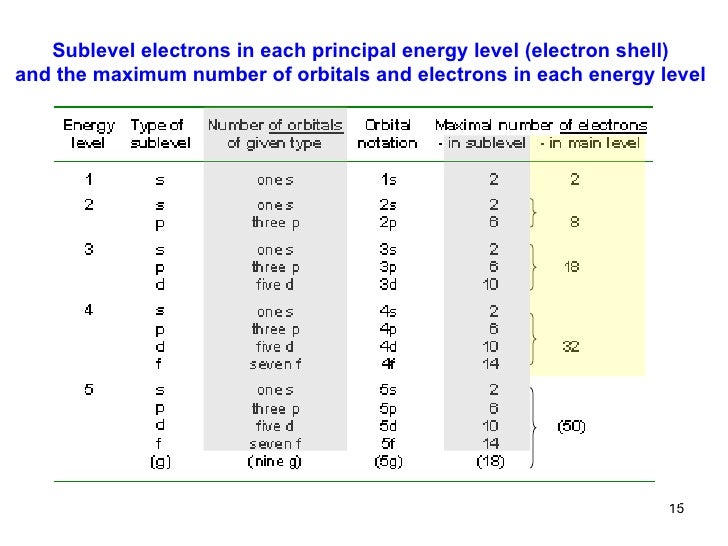
Maximum number of electrons in a subshell formula. Therefore the first shell can hold only 2 electrons the second 8 then 18 25 32 and 50. Each d subshell holds at most 10 electrons. Each p subshell holds at most 6 electrons.
Of orbitals in various subshell are. Each f subshell holds at most 14 electrons. Using the above you can work out the maximum number of electrons that can occupy a shell is 2n 2.
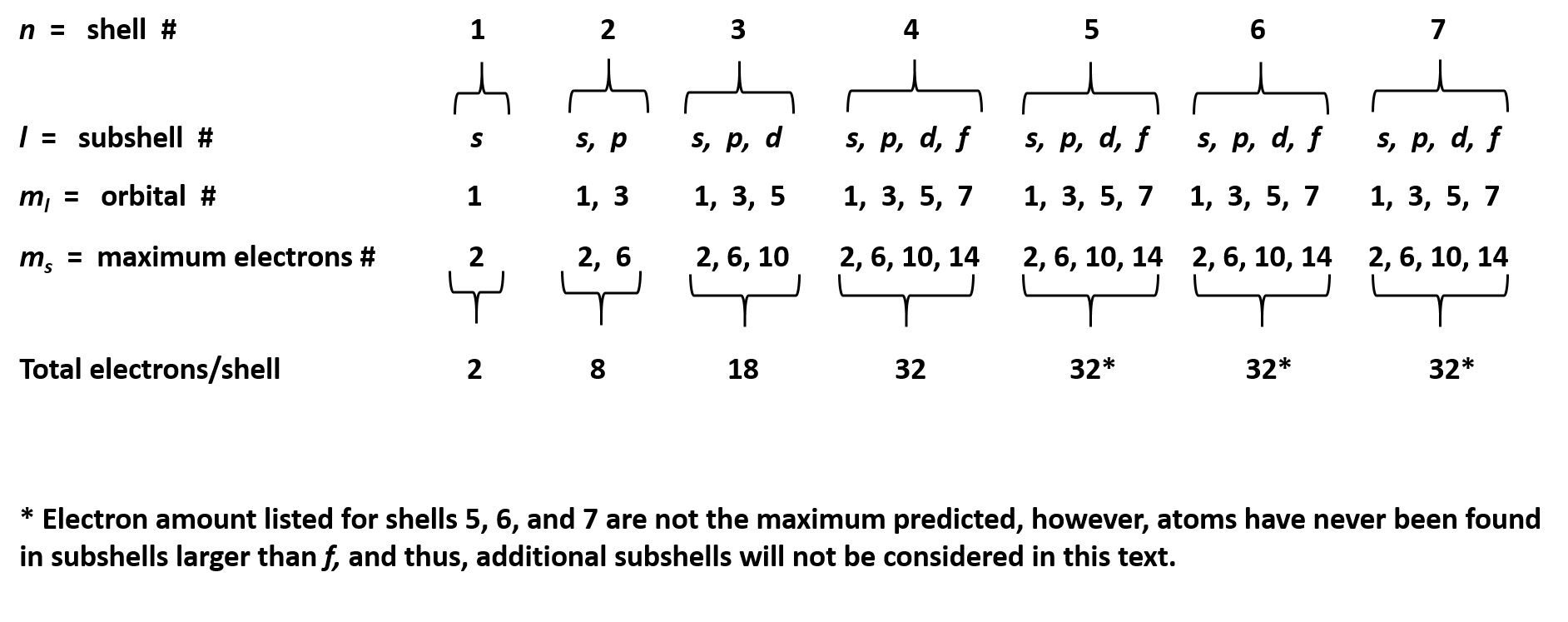
Are solved by group of students and teacher of neet which is also the largest student community of neet. This model breaks down at the n 3 shell because each shell has subshells. The maximum number of electrons that can be held in each shell can be calculated using the formula 2n 2 where n is the shell number.
In general the nth shell can hold up to 2n 2 electrons. Can you explain this answer. S has 1 orbital.
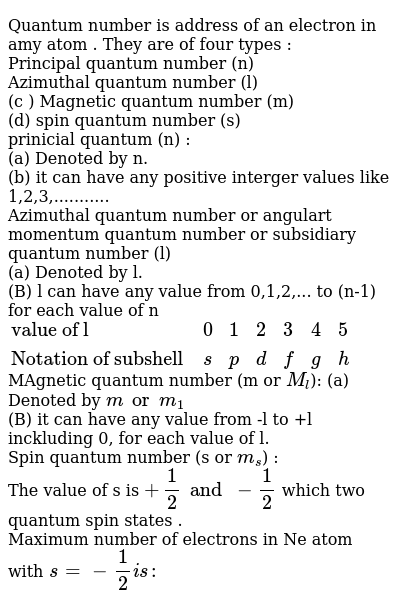
Total number of subshells 2l 1 maximum number electron in a subshell 2 2l 1 4l 2 in view of the coronavirus pandemic we are making live classes and video classes completely free to prevent interruption in studies. The questions and answers of the maximum number of electrons in a subshell is given by the expressiona 4l 2b 4l 2c 2l 1d 2n2correct answer is option b. Therefore the k shell which contains only an s subshell can hold up to 2 electrons.
For n 2 we have only s p orbitals. Of electrons 4 3 12. Each shell must be full before the next starts to fill.
P has 3 orbital. The l shell which contains an s and a p can hold up to 2 6 8 electrons and so forth. It is given that each orbital can have a maximum of 3 electrons.
Option d is correct. Electrons are placed into available shells starting with the lowest energy level.
 Electron Configuration Vce Chemistry
Electron Configuration Vce Chemistry
 How Many Electrons Are In Each Energy Level
How Many Electrons Are In Each Energy Level
 Structure Of Solid Materials Engineering
Structure Of Solid Materials Engineering

 Quantum Numbers For The First Four Shells Video Khan Academy
Quantum Numbers For The First Four Shells Video Khan Academy
 Ch150 Chapter 2 Atoms And Periodic Table Chemistry
Ch150 Chapter 2 Atoms And Periodic Table Chemistry
 The Pauli Exclusion Principle Physics
The Pauli Exclusion Principle Physics
 Maximum Number Of Electrons In A Subshell With L 3 And N 4
Maximum Number Of Electrons In A Subshell With L 3 And N 4
 A How Many Sub Shell Are Associated With N 4 B How Many
A How Many Sub Shell Are Associated With N 4 B How Many
 Electronic Structure Of Atoms Electron Configurations
Electronic Structure Of Atoms Electron Configurations
Electron Configuration Chemistry 10
 Openstax College Physics Solution Chapter 30 Problem 45
Openstax College Physics Solution Chapter 30 Problem 45
 The Maximum Number Of Electrons In A Subshell Is Given By The
The Maximum Number Of Electrons In A Subshell Is Given By The
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcqt2vnp334n00dq106eyub35tkswrezxrwns Gfsceyqmpcnpdn Usqp Cau
 Ppt Electron Configurations And Orbital Diagrams Powerpoint
Ppt Electron Configurations And Orbital Diagrams Powerpoint
 The Maximum Number Of Electrons In A Subshell Is Given By The Expr
The Maximum Number Of Electrons In A Subshell Is Given By The Expr
 Aleks Calculating The Capacity Of Electron Subshells Youtube
Aleks Calculating The Capacity Of Electron Subshells Youtube
How Many Electrons Can Fit In The Orbital For Which N 3 And L 1
Electronic Structure And Periodic Table Mcat Review
 What Is The Maximum Number Of Electrons In A Subshell That Can
What Is The Maximum Number Of Electrons In A Subshell That Can
 Maximum Number Of Electrons In A Subshell With L 3 And N 4 Is
Maximum Number Of Electrons In A Subshell With L 3 And N 4 Is
 5 What Is The Maximum Number Of Electrons That Can Occupy A 4f
5 What Is The Maximum Number Of Electrons That Can Occupy A 4f
 The Maximum Number Of Electrons In A Subshell Having The Same Val
The Maximum Number Of Electrons In A Subshell Having The Same Val
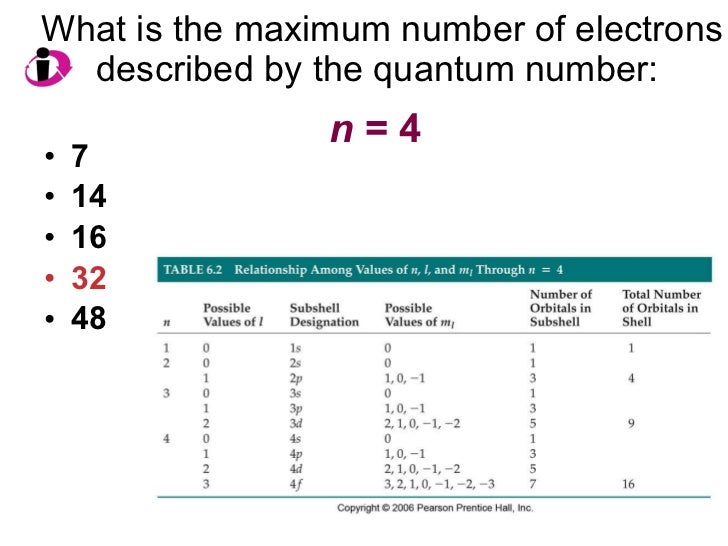 Chapter 6 Lecture Electrons In Atoms
Chapter 6 Lecture Electrons In Atoms
 The Pauli Exclusion Principle Physics
The Pauli Exclusion Principle Physics
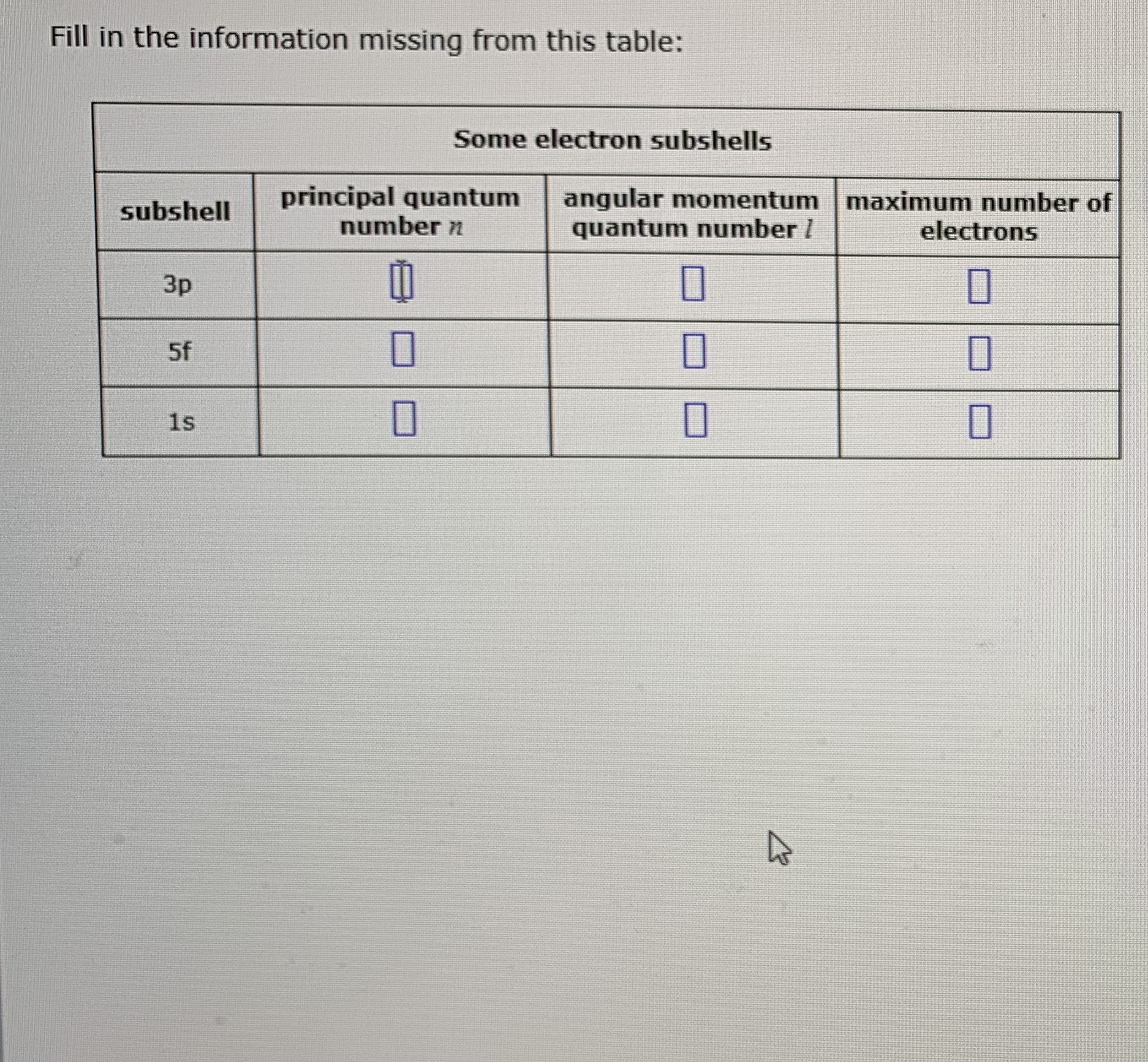 Answered Fill In The Information Missing From Bartleby
Answered Fill In The Information Missing From Bartleby
 Fill In The Information Missing From This Table Some Electron
Fill In The Information Missing From This Table Some Electron
 Electron Orbital Definition Shells Shapes Video Lesson
Electron Orbital Definition Shells Shapes Video Lesson
 Chemistry Review Electron Shells And Subshells Free Homework Help
Chemistry Review Electron Shells And Subshells Free Homework Help
 30 9 The Pauli Exclusion Principle College Physics Openstax
30 9 The Pauli Exclusion Principle College Physics Openstax
 Ground State Electron Configuration Definition Example Video
Ground State Electron Configuration Definition Example Video
How Many Electrons Can Fit In The Orbital For Which N 3 And L 1
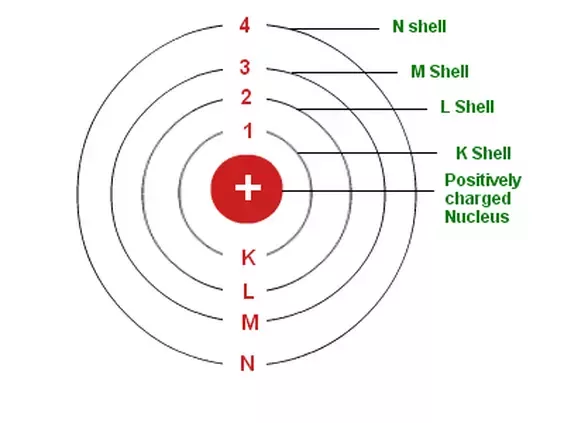 Why Is The Maximum Number Of Electrons In A Shell Fixed By 2n 2
Why Is The Maximum Number Of Electrons In A Shell Fixed By 2n 2
 What Are The Maximum Number Of Electrons In Each Shell
What Are The Maximum Number Of Electrons In Each Shell
 Fill In The Information Missing From This Table Subshell 4
Fill In The Information Missing From This Table Subshell 4
 Columni Columnii A Number Of Orbitials In Then N
Columni Columnii A Number Of Orbitials In Then N


Posting Komentar
Posting Komentar