According To The Kinetic Molecular Theory
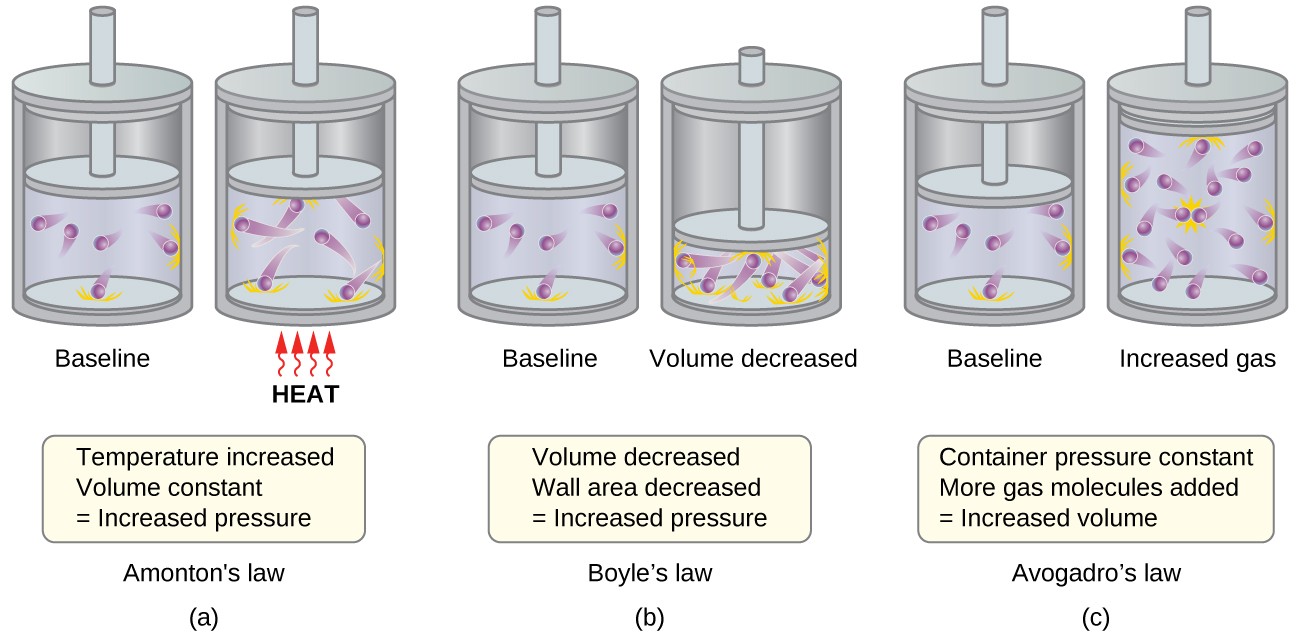
The gas molecules collide more frequently and more energetically with the wall according to kinetic molecular theory if the temperature of a gas is raised from 100 c to 200 c the average kinetic energy of the gas will. Solids liquids and gases.

An ideal gas is a hypothetical gas.

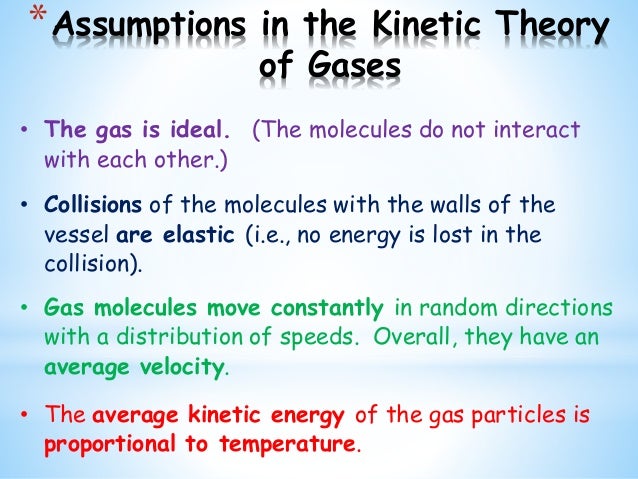
According to the kinetic molecular theory. The kinetic molecular theory allows us to explain the existence of the three phases of matter. The kinetic molecular theory predicts that pressure rises as the temperature of a gas increases because. Collisions with the walls account for the pressure of the.
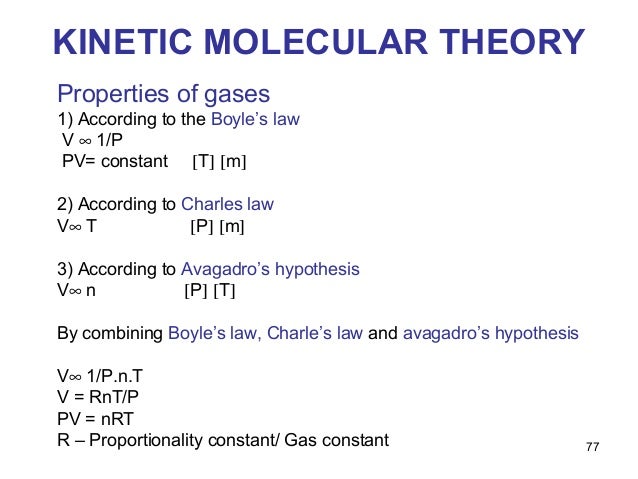
This can be expressed with the following equation where k represents the boltzmann constant. The kinetic theory of gases is a historically significant but simple model of the thermodynamic behavior of gases with which many principal concepts of thermodynamics were established the model describes a gas as a large number of identical submicroscopic particles atoms or molecules all of which are in constant rapid random motion their size is assumed to be much smaller than the. The kinetic molecular theory can be used to explain the results graham obtained when he studied the diffusion and effusion of gases.
That conforms to all of the assumptions of the kinetic theory. Most of the volume which a gas occupies is empty space 2 gas molecules are in constant random motion. The key to this explanation is the last postulate of the kinetic theory which assumes that the temperature of a system is proportional to the average kinetic energy.
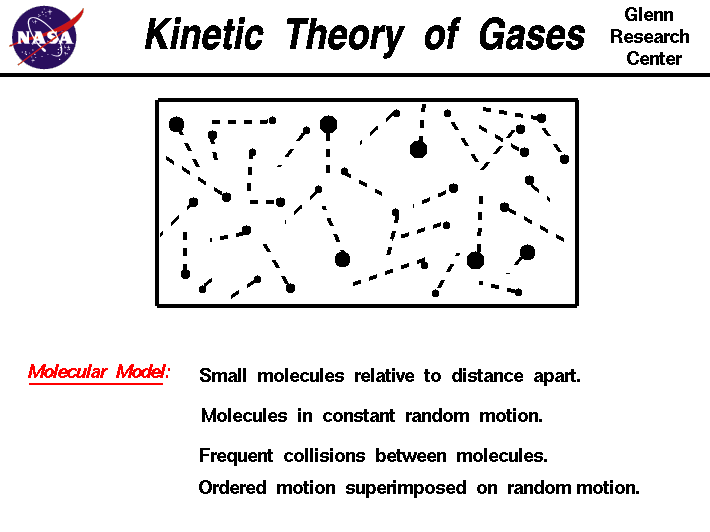
Postulates of kinetic theory of gases 1 the molecules in a gas are small and very far apart. In this model the submicroscopic particles atoms or molecules that make up the gas are continually moving around in random motion constantly colliding not only with each other but also with the sides of any container that the gas is. Just as many molecules are moving in one direction as in any other 3 molecules can collide with each other and with the walls of the container.
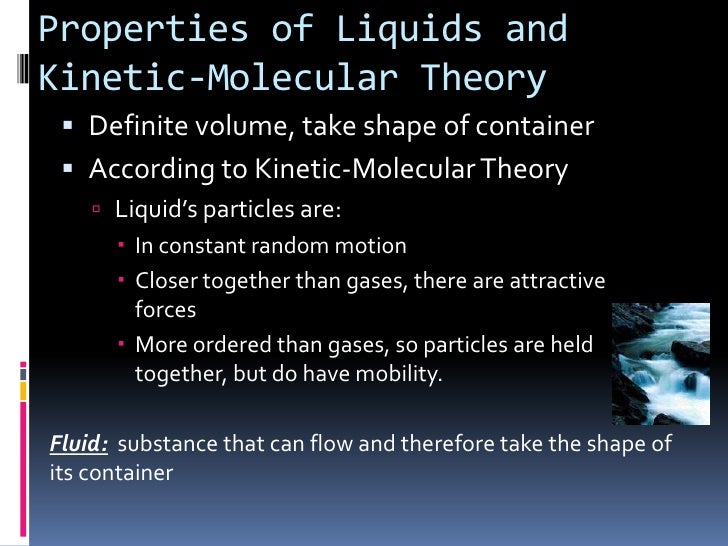
Solid liquid and gas. According to the kinetic molecular theory all gaseous particles are in constant random motion at temperatures above absolute zero. In addition it helps explain the physical characteristics of each phase and how phases change from one to another.
The kinetic molecular theory is essential for the explanations of gas pressure compressibility diffusion and mixing. Figure 6 8 distribution of molecular speeds oxygen gas at 100 20 and 600 c 1 according to the kinetic molecular theory the average kinetic energy of gas particles is proportional to the absolute temperature of the gas. The movement of gaseous particles is characterized by straight line trajectories interrupted by collisions with other particles or with a physical boundary.
The kinetic molecular theory and graham s laws. The kinetic theory of gases is a scientific model that explains the physical behavior of a gas as the motion of the molecular particles that compose the gas. The kinetic molecular theory explains the behavior of.
According to the kinetic molecular theory gases condense into liquids because of.
 Gases The Kinetic Molecular Theory Of Gases A Gas Consists Of
Gases The Kinetic Molecular Theory Of Gases A Gas Consists Of
 Chem Ii Kinetic Molecular Theory Of Gases Liquids And Solids
Chem Ii Kinetic Molecular Theory Of Gases Liquids And Solids
 Chapter 10 States Of Matter The Kinetic Molecular Theory
Chapter 10 States Of Matter The Kinetic Molecular Theory
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctbvxrvrwcakqm0fru6lhybdw B91tp5y55 Wqlu2zfapkk3lkm Usqp Cau
 Which Of The Following Statements Is Are T Clutch Prep
Which Of The Following Statements Is Are T Clutch Prep
 Solved 2 According To The Kinetic Molecular Theory What
Solved 2 According To The Kinetic Molecular Theory What
 Answered Question 13 According To The Kinetic Bartleby
Answered Question 13 According To The Kinetic Bartleby
 Chemistry Phases Of Matter And Kinetic Molecular Theory Released
Chemistry Phases Of Matter And Kinetic Molecular Theory Released
 13 1 Kinetic Molecular Theory Chemistry Libretexts
13 1 Kinetic Molecular Theory Chemistry Libretexts
 Solved Question 10 0 25 Points Saved According To The K
Solved Question 10 0 25 Points Saved According To The K
 Solved Metropcs 0 40 D 12 05 Am A Bbhosted Cuny Edu
Solved Metropcs 0 40 D 12 05 Am A Bbhosted Cuny Edu
 Chapter 10 States Of Matter Ppt Download
Chapter 10 States Of Matter Ppt Download
 Solved Question 10 According To The Kinetic Molecular The
Solved Question 10 According To The Kinetic Molecular The

5 6 Kinetic Molecular Theory Chemistry Libretexts
 Stereo Chemistry And Kinetic Molecular Theory
Stereo Chemistry And Kinetic Molecular Theory
 Unit 3 States Of Matter Practice Exam Pdf Free Download
Unit 3 States Of Matter Practice Exam Pdf Free Download
 Solved Question 4 According To The Kinetic Molecular Theo
Solved Question 4 According To The Kinetic Molecular Theo
 The Kinetic Molecular Theory Chemistry For Majors
The Kinetic Molecular Theory Chemistry For Majors
 The Kinetic Molecular Theory Of Gases Ppt Download
The Kinetic Molecular Theory Of Gases Ppt Download
 Question 33 Which Statement Is True According To The Kinetic
Question 33 Which Statement Is True According To The Kinetic
 According To The Kinetic Molecular Theory The Pressu
According To The Kinetic Molecular Theory The Pressu
 Solved According To The Kinetic Molecular Theory Which O
Solved According To The Kinetic Molecular Theory Which O
 Image Result For Kinetic Molecular Theory High School Chemistry
Image Result For Kinetic Molecular Theory High School Chemistry
 Chapter 10 Test Review Sheet 1 The Kinetic Molecular Theory Of
Chapter 10 Test Review Sheet 1 The Kinetic Molecular Theory Of

 Solved Part A Which Of The Following Statements Describes
Solved Part A Which Of The Following Statements Describes
 Chapter 13 States Of Matter 13 1 The Nature Of Gases Kinetic
Chapter 13 States Of Matter 13 1 The Nature Of Gases Kinetic
 2014 Pearson Education Inc Basic Chemistry 4 E Chapter 11
2014 Pearson Education Inc Basic Chemistry 4 E Chapter 11
 According To Kinetic Molecular Theory Kmt Clutch Prep
According To Kinetic Molecular Theory Kmt Clutch Prep

 Wilhelm Wien According To The Kinetic Theory Of Gases The Mean
Wilhelm Wien According To The Kinetic Theory Of Gases The Mean
 Characteristics Of Gases The Kinetic Molecular Theory Of Matter
Characteristics Of Gases The Kinetic Molecular Theory Of Matter
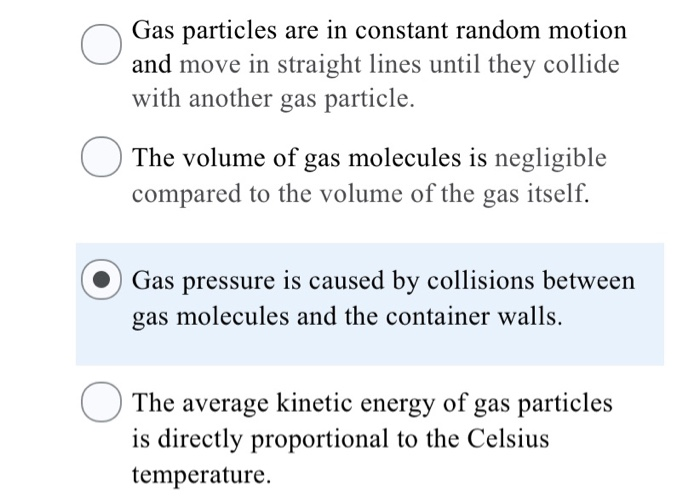 Solved According To The Kinetic Molecular Theory Which O
Solved According To The Kinetic Molecular Theory Which O
 Chapter 10 Notes The Kinetic Molecular Theory Is Based On
Chapter 10 Notes The Kinetic Molecular Theory Is Based On


Posting Komentar
Posting Komentar