Which Unit Can Be Used To Express Solution Concentration
This is how many mol there are in each liter of the solution. That is we need specific units of concentration in this section we will introduce several common and useful units of concentration.
 Expressing Concentration Of Solutions Methods Formulas Videos Q A
Expressing Concentration Of Solutions Methods Formulas Videos Q A
Molality m and molarity m both express the concentration of a chemical solution.
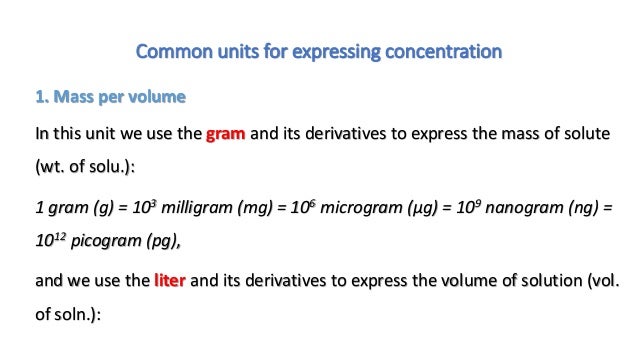
Which unit can be used to express solution concentration. Since the numerator and denominator have different units this concentration unit is not a true relative unit e g. Molarity is the number of moles of solute per liter of solution. Which unit can be used to express solution concentration.
It can be. In other words you can t understand how to convert and use information regarding solutions unless we first discuss the units in which the concentration of said solutions will be expressed. Rather than qualitative terms section 11 1 some definitions we need quantitative ways to express the amount of solute in a solution.
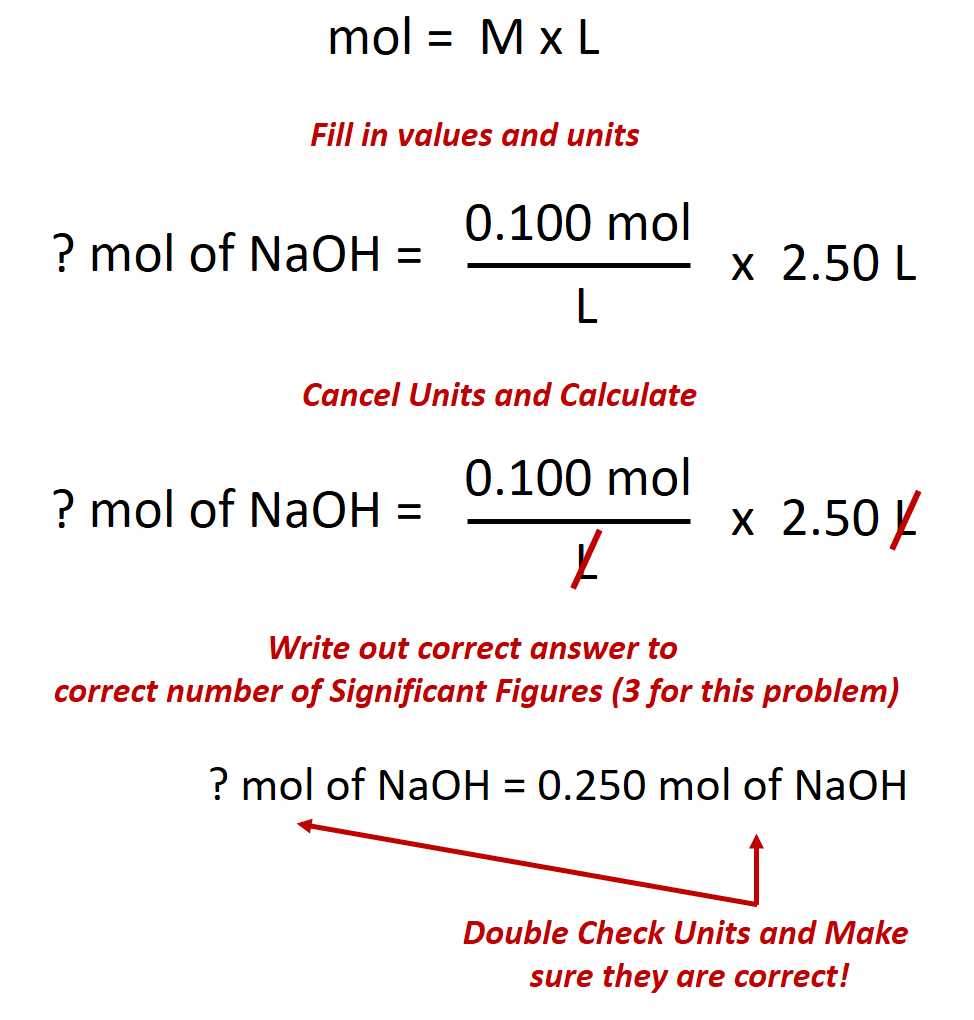
D moles of nacl per liter of solution 3 the molarity of an aqueous solution of nacl is defined as the a j mol b l mol c mol l d mol s 4 which unit can be used to express solution concentration. In lehman s terms it is the amount of the stuff put into the solution in one liter. The concentration of a solution is the quantity of solute in a given quantity of solution.
Percentage however it is often used as an easy concentration unit since volumes of. A 6 0 mol b 8 0 mol c 1 0 mol d 0 70 mol 5 what is the total number of moles of nacl s needed to make 3 0 liters of a 2 0 m nacl solution. If the solvent is water and the concentration of solute is fairly low i e dilute solution molality and molarity are approximately the same.
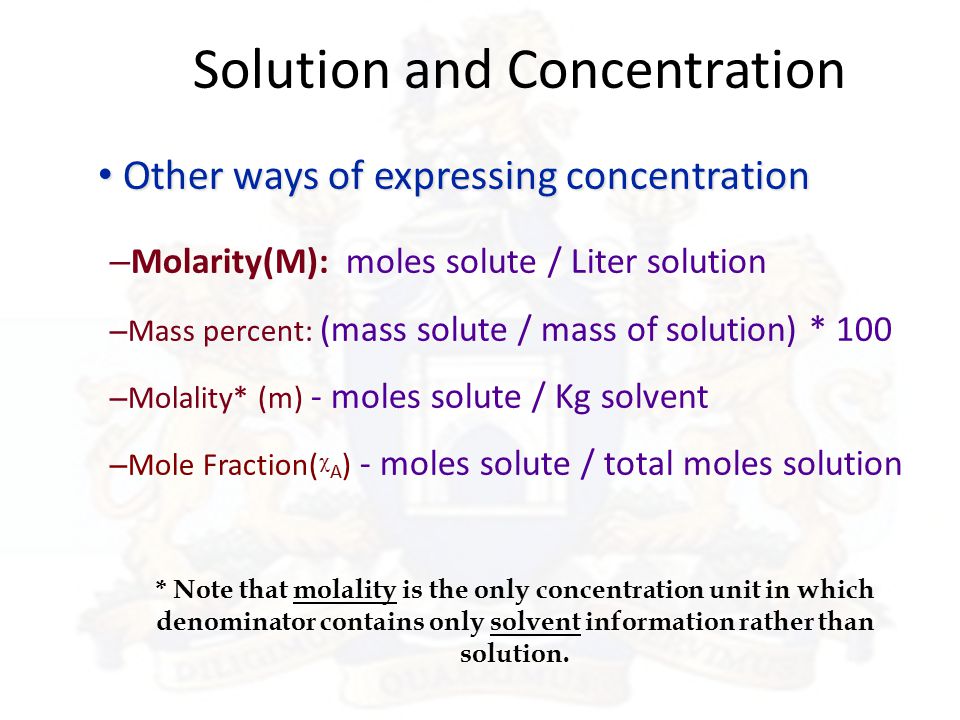
The most common units are molarity molality normality mass percent volume percent and mole fraction. Different units are used to express the concentrations of a solution depending on the application. Solution concentration is an important underlying concept that you should know well before we start the next few lectures on solutions.
Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution there are multiple units of concentration which unit you use depends on how you intend to use the chemical solution. Molality is the number of moles of solute per kilogram of solvent. The mass volume percent is used to express the concentration of a solution when the mass of the solute and volume of the solution is given.
What is a unit often used to express the concentration of a solution in 1 divided by 1000000000000. Which unit can be used to express solution concentration. 1 j mol 2 l mol 3 mol l 4 mol s get the answers you need now.
Which unit can be used to express solution concentration. Molarity m is defined as the number of moles of solute divided by the number of liters of solution. Can a country survive and prosper without.
 Ch104 Chapter 7 Solutions Chemistry
Ch104 Chapter 7 Solutions Chemistry
 Solutions And Their Concentrations In Analytical Chemistry By Azad Al
Solutions And Their Concentrations In Analytical Chemistry By Azad Al
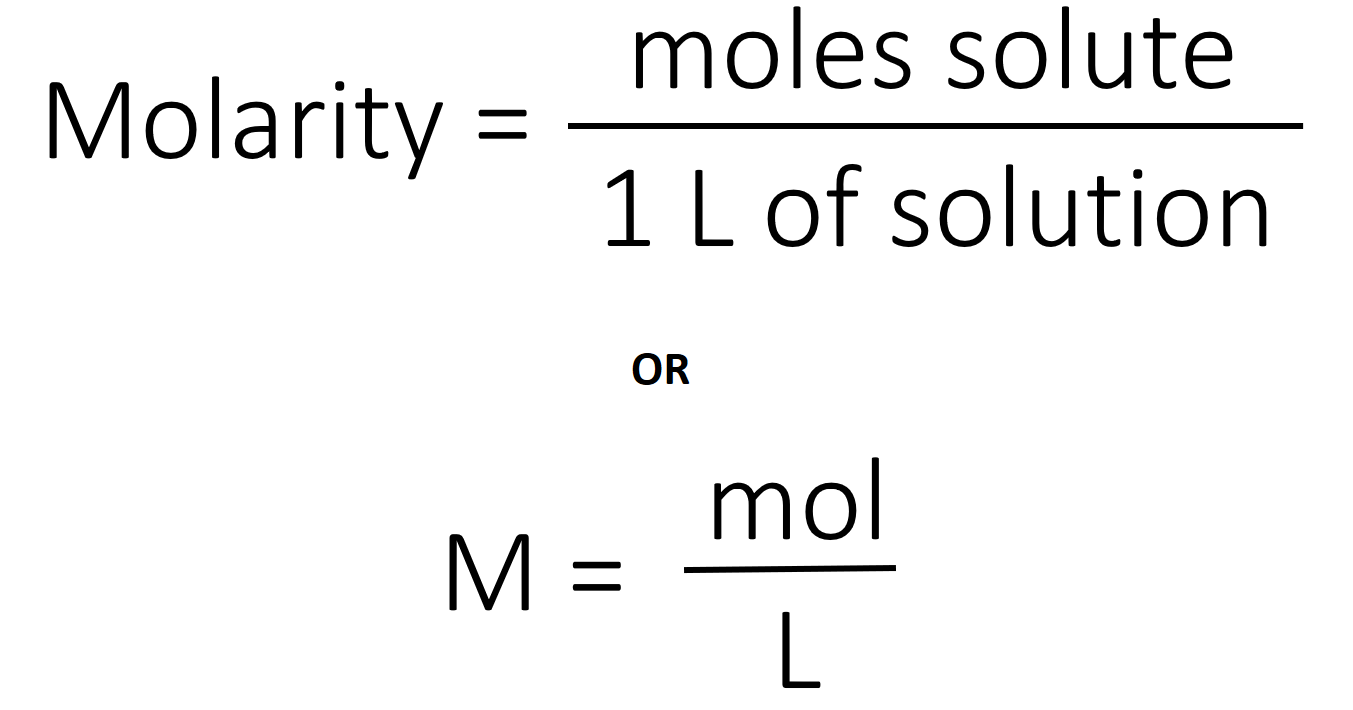 Ch104 Chapter 7 Solutions Chemistry
Ch104 Chapter 7 Solutions Chemistry
/scientist-examining-the-sample-in-a-conical-flask-140670921-58d528153df78c516218950b.jpg) Concentration Definition Chemistry
Concentration Definition Chemistry
Https Slcc Instructure Com Courses 394267 Files 65360687 Download Wrap 1
 Solutions Chemistry Properties Of Solutions L Objectives
Solutions Chemistry Properties Of Solutions L Objectives
 Solved 5 1 6 Pts A Solution Has 5 75 Micrograms Of Cu2
Solved 5 1 6 Pts A Solution Has 5 75 Micrograms Of Cu2
 13 4 Expressing Concentration Youtube
13 4 Expressing Concentration Youtube
 Solutions And Their Concentrations In Analytical Chemistry By Azad Al
Solutions And Their Concentrations In Analytical Chemistry By Azad Al
/480811109-56a12f5b3df78cf772683a9d.jpg) Why Is Molality Used Instead Of Molarity
Why Is Molality Used Instead Of Molarity
 Ch 12 1 Types Of Mixtures Ppt Video Online Download
Ch 12 1 Types Of Mixtures Ppt Video Online Download
Https Nroer Gov In Media C A A 67b908bc0bb3148493c4f03012377e283696fc7a5de84975df3c7971866c9 Pdf
 Can Be Used To Express The Concentration Of A Solution لم يسبق له
Can Be Used To Express The Concentration Of A Solution لم يسبق له
 Ch150 Chapter 7 Solutions Chemistry
Ch150 Chapter 7 Solutions Chemistry
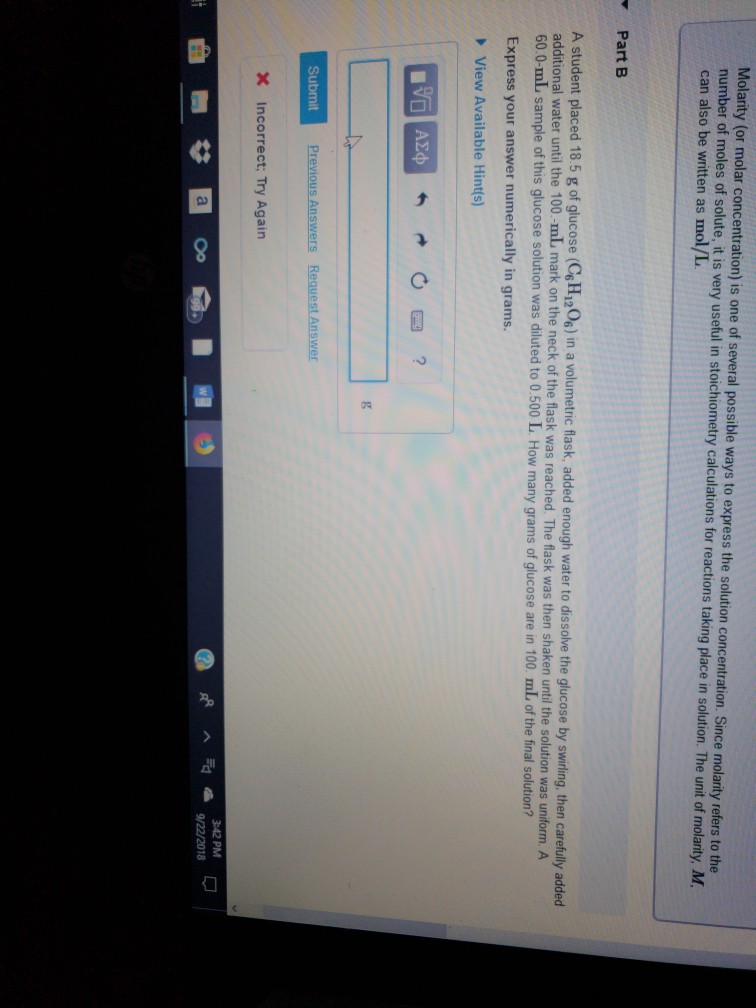
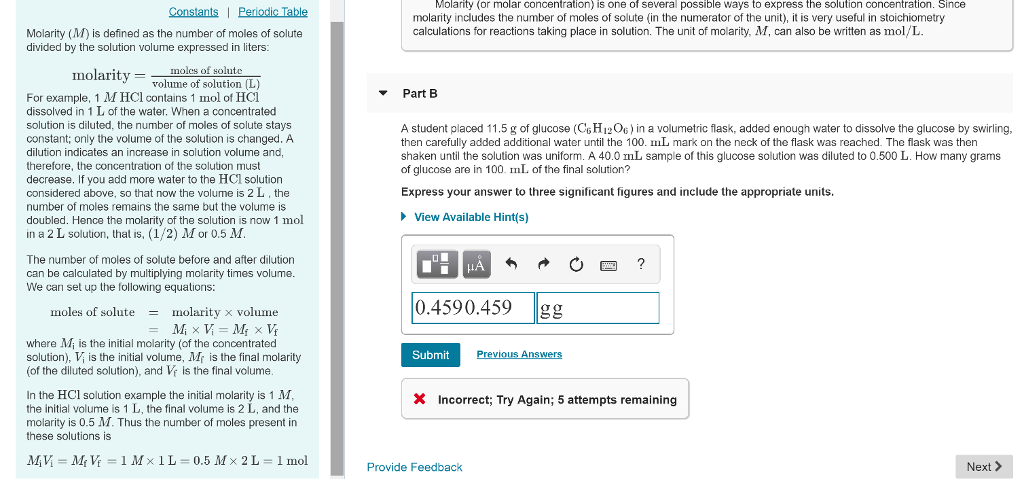 Solved Molarity Or Molar Concentration Is One Of Severa
Solved Molarity Or Molar Concentration Is One Of Severa
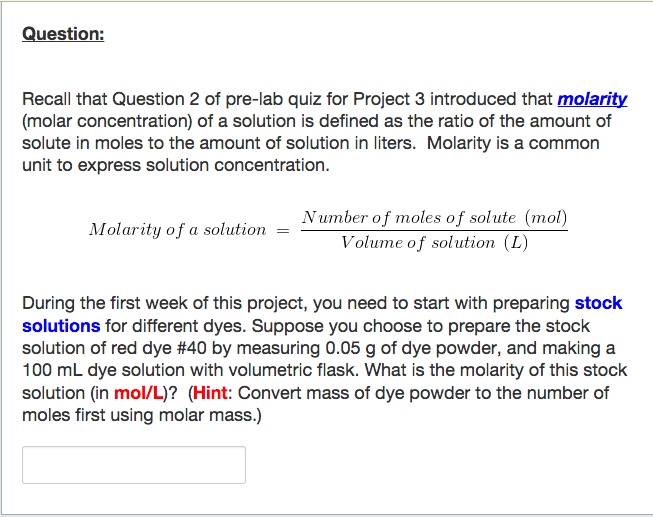 Solved Question Recall That Question 2 Of Pre Lab Quiz F
Solved Question Recall That Question 2 Of Pre Lab Quiz F
 Ch104 Chapter 7 Solutions Chemistry
Ch104 Chapter 7 Solutions Chemistry
Http Web Iyte Edu Tr Serifeyalcin Lectures Chem201 Cn 4 Pdf
 Solution Concentration Chemistry Ways To Express Solution
Solution Concentration Chemistry Ways To Express Solution
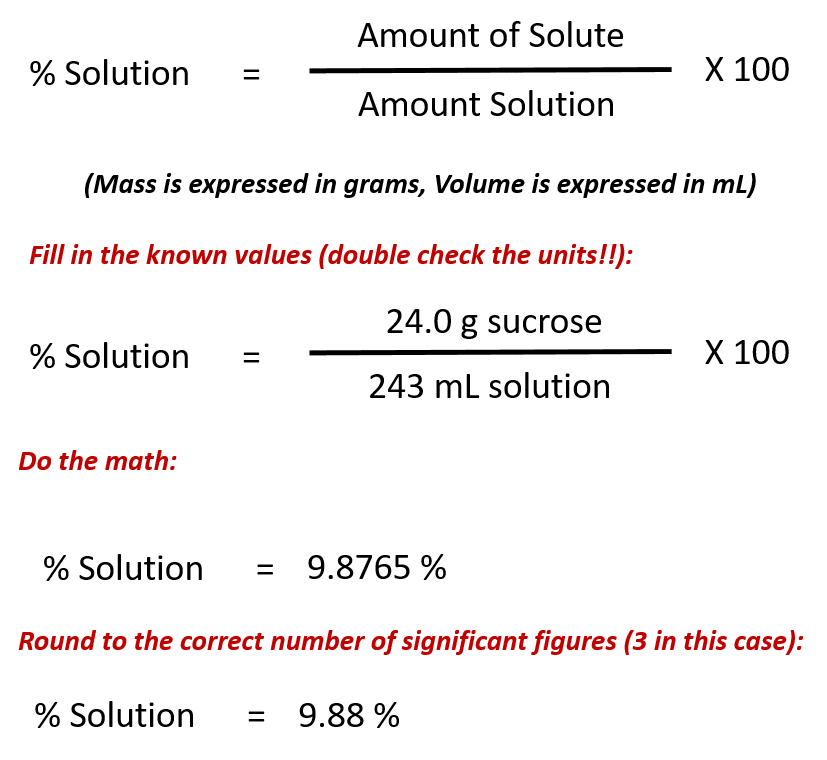 Ch104 Chapter 7 Solutions Chemistry
Ch104 Chapter 7 Solutions Chemistry
 Common Relative Expressions Of Concentration Units Download Table
Common Relative Expressions Of Concentration Units Download Table
/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png) How To Calculate Normality Of A Solution
How To Calculate Normality Of A Solution
 Solutions Solution Homogeneous Mixture Ppt Download
Solutions Solution Homogeneous Mixture Ppt Download
 How To Calculate Solution Concentration In Molarity And Percent By
How To Calculate Solution Concentration In Molarity And Percent By
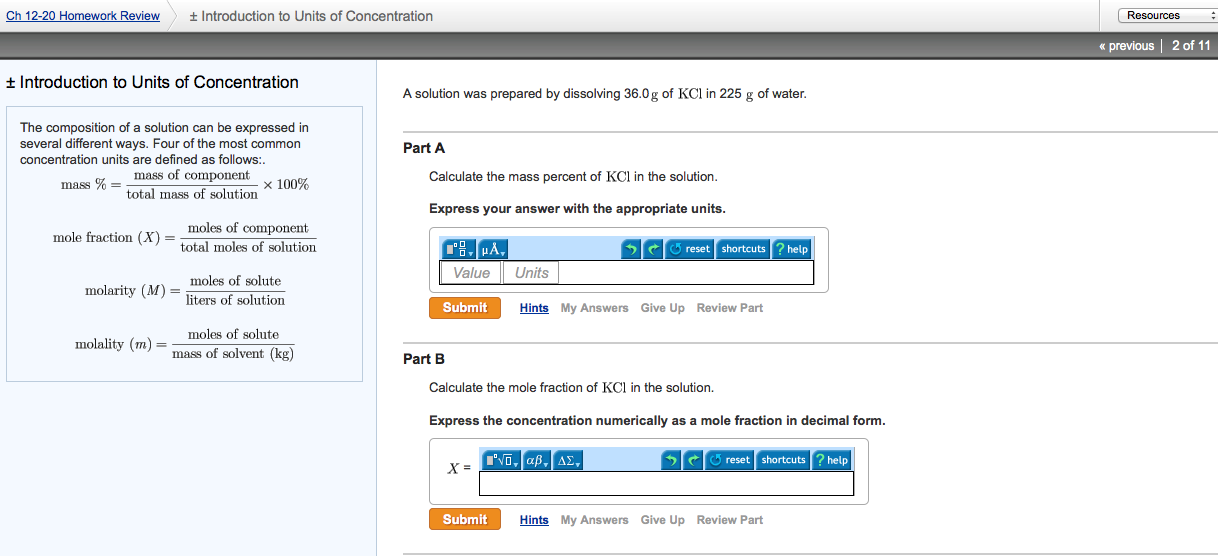 Solved The Composition Of A Solution Can Be Expressed In
Solved The Composition Of A Solution Can Be Expressed In
 Solution Concentration Chemistry Master
Solution Concentration Chemistry Master
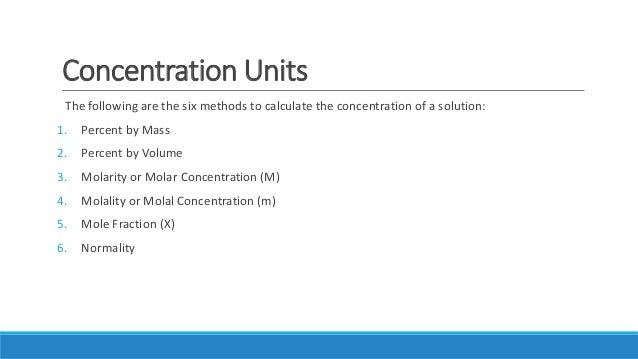 Quantitative Expressions Of The Concentration Of Solutions
Quantitative Expressions Of The Concentration Of Solutions
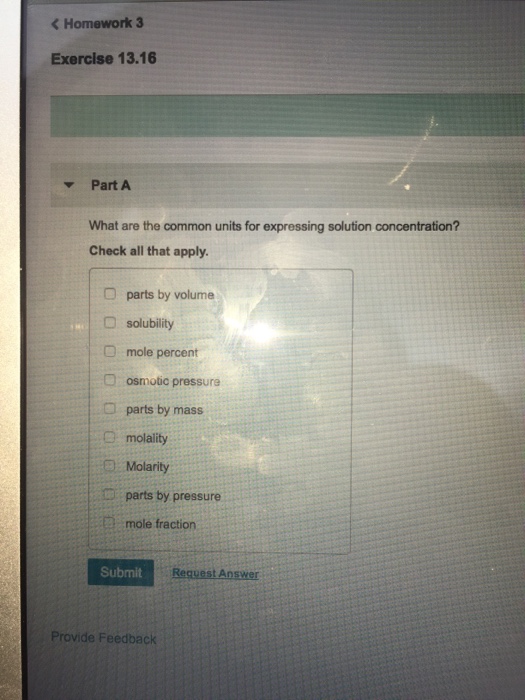
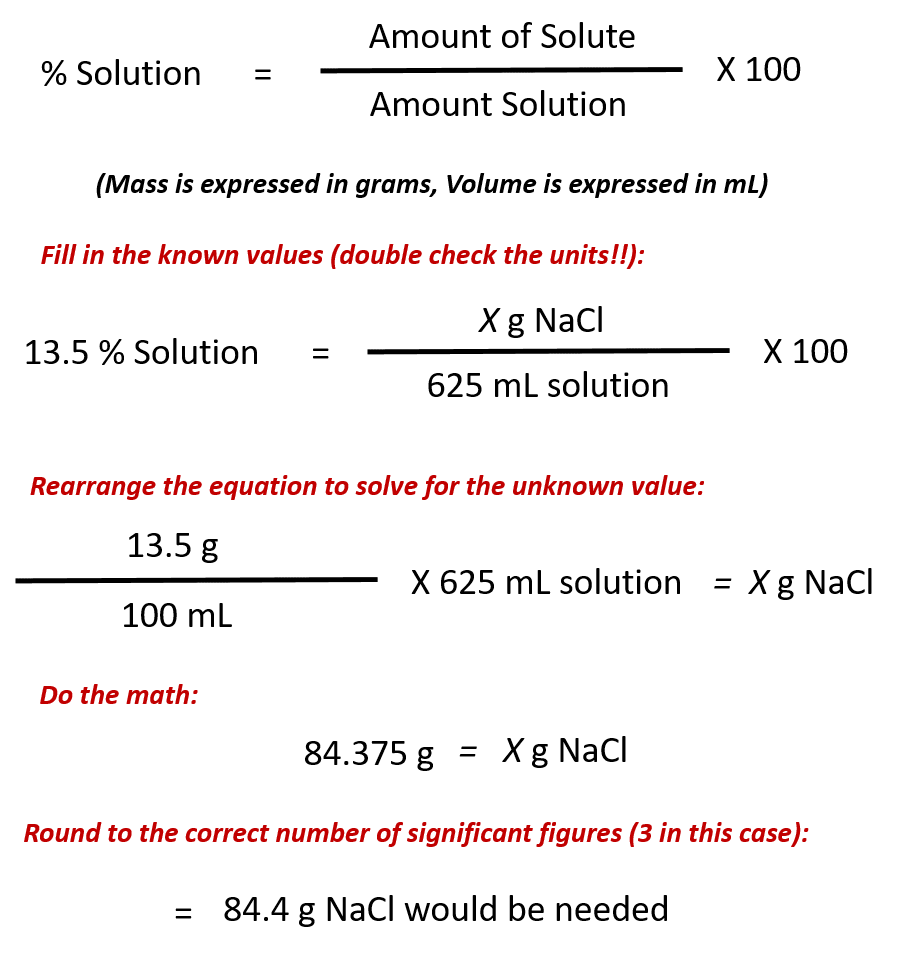 Ch104 Chapter 7 Solutions Chemistry
Ch104 Chapter 7 Solutions Chemistry
 Solutions Solution Homogeneous Mixture Ppt Download
Solutions Solution Homogeneous Mixture Ppt Download
 How To Calculate Solution Concentration In Molarity And Percent By
How To Calculate Solution Concentration In Molarity And Percent By
/173637959-58befc655f9b58af5c9acab8.jpg) How To Calculate Concentration
How To Calculate Concentration
 Percent Concentration Calculation Part 03 Volume By Volume V V
Percent Concentration Calculation Part 03 Volume By Volume V V
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcrv5qioyjlfrrpgs3qgzen7uv1shsnek2lwiwrr56pod2ximbkn Usqp Cau
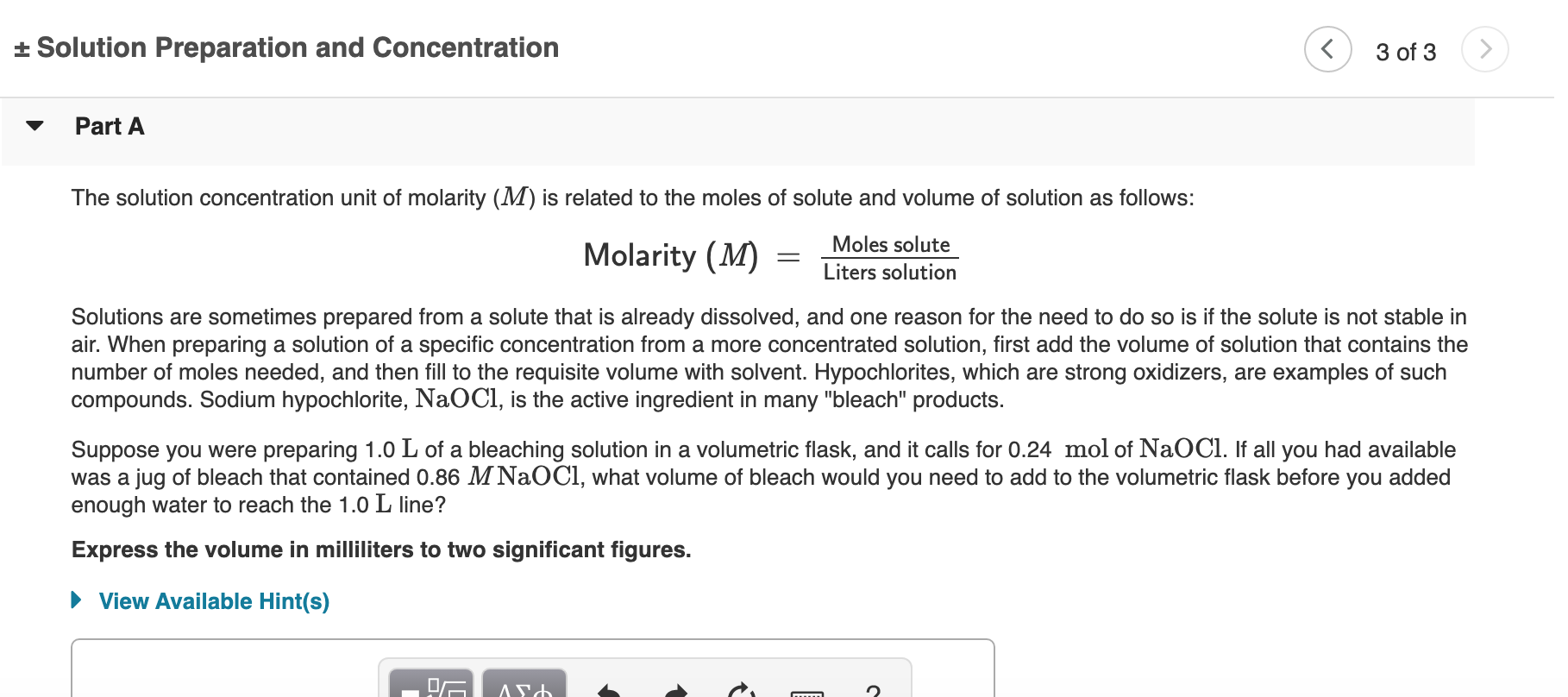 Solved Solution Preparation And Concentration C 3 Of 3
Solved Solution Preparation And Concentration C 3 Of 3


Posting Komentar
Posting Komentar