What Is Raoult S Law Formula
You can use the simplified definition in the box below in the case of a single volatile liquid the solvent and a non volatile solute. Raoult s law there are several ways of stating raoult s law and you tend to use slightly different versions depending on the situation you are talking about.
 Applications Applications Of Raoult S Law Ppt Video Online Download
Applications Applications Of Raoult S Law Ppt Video Online Download
Why raoult s law works.
What is raoult s law formula. We will further take an in depth look at raoult s law and understand the principle behind the law as well as its application and limitations in this lesson. Raoult s law was established in the year 1887 and is also considered as the law of thermodynamics. Read this article on raoult s law and understand the concept of raoult s law for volatile liquids and non volatile solutes.
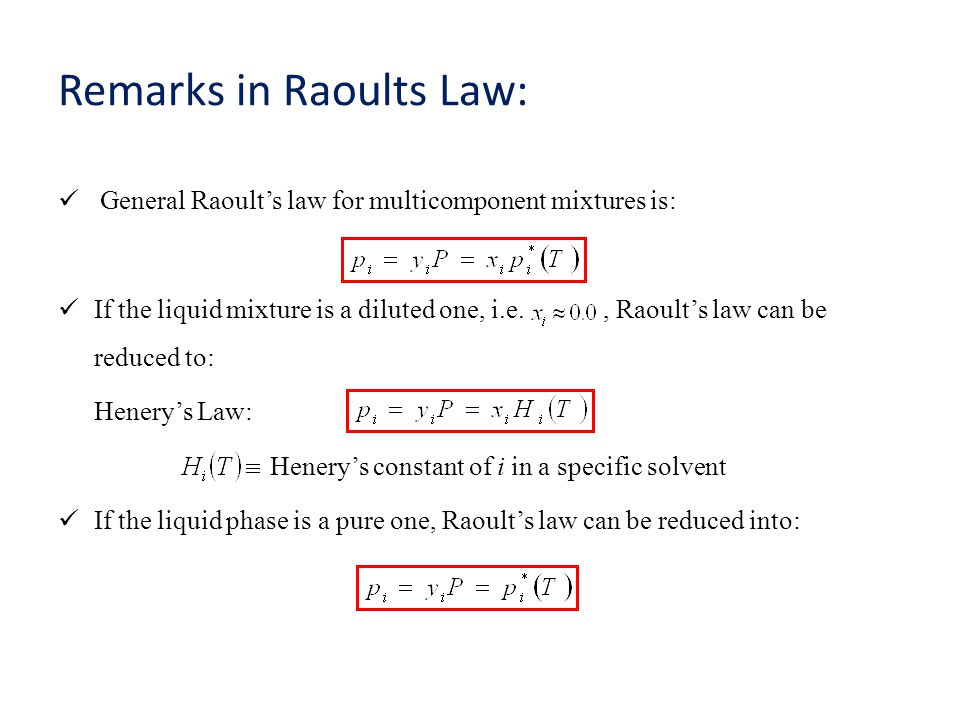
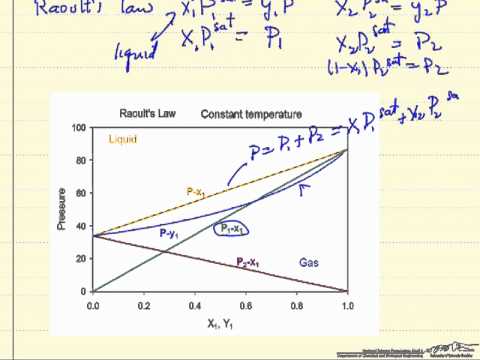
A closed vessel containing a binary solution of two volatile liquids an equilibrium will be established between the vapor and liquid phase due to the evaporation of its components. Vapor pressure of the pure substance i in a solution with two liquids a and b if no gas are present the total vapor pressure is given by. Raoult s law is expressed by the formula.
So ϒ ab the attractive force between the molecule a and b. Mole fraction in the solution. Suppose a and b are the molecule of solvent and solute.
If you look review the concepts of colligative properties you will find that adding a solute lowers vapor pressure because the additional solute particles will fill the gaps between the solvent particles and take up space. For example we can generate a pxy diagram by fixing the temperature hence the two pure component vapor pressures at the desired value. ϒ aa the attractive force between the molecule a and a.
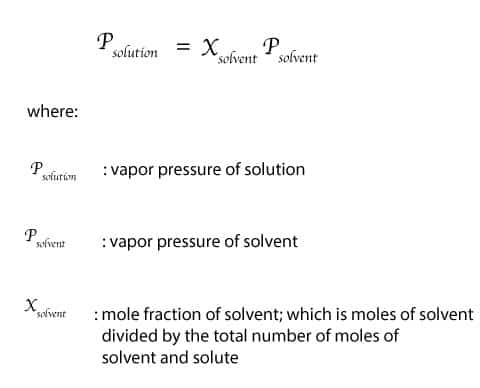
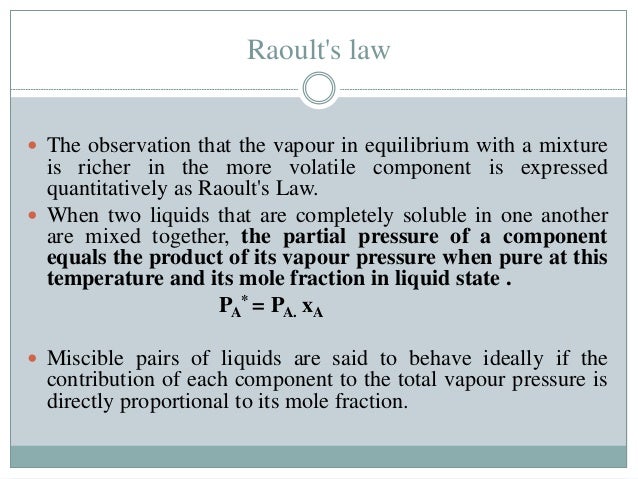
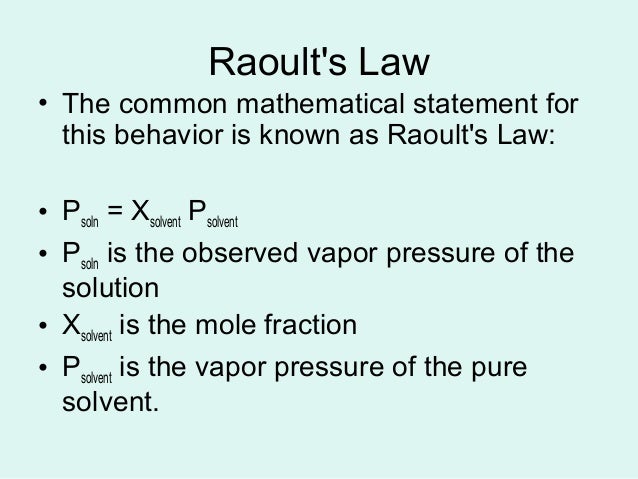
P solution χ solvent p 0 solvent where p solution is the vapor pressure of the solution χ solvent is mole fraction of the solvent p 0 solvent is the vapor pressure of the pure solvent if more than one solute is added to the solution each individual solvent s component is added to the total pressure. In consequence the relative lowering of vapour pressure of a dilute solution. Raoult s law for ideal solution.
This means less of the solvent will be on the surface and less will be able to break free to enter the gas phase resulting in a lower vapor pressure. It states that the partial pressure of each component of an ideal mixture of liquids is equal to the vapour pressure of the pure component multiplied by its mole fraction in the mixture. In fact those diagrams were generated from raoult s law.
Raoult s law is given by. Raoult s law ˈ r ɑː uː l z law is a law of thermodynamics established by french chemist françois marie raoult in 1887. A solution which strictly obeys raoult s law is known as ideal solution.
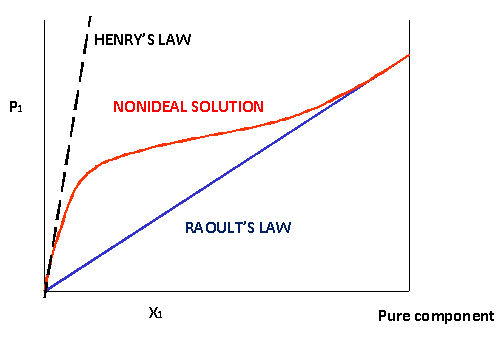
Raoult s law can be used to solve vle problems in place of the txy and pxy diagrams that we used on the last two pages. P solution χ solvent p 0 solvent where p solution is the vapor pressure of the solution χ solvent is mole fraction of the solvent p 0 solvent is the vapor pressure of the pure solvent when two or more volatile solutions are mixed each pressure component of the mixed solution is added together to find the total vapor pressure. A non ideal solution shows deviation from raoult s law.
Pressure of component i x i. Raoult s law is expressed by the vapor pressure equation. Learning the concept will help in your jee main and jee advanced exam preparations.
 Raoult S Law Vapor Pressure Partial Pressure Of Volatile
Raoult S Law Vapor Pressure Partial Pressure Of Volatile
 How Does The Vapor Pressure Of Solution Depend On The
How Does The Vapor Pressure Of Solution Depend On The
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcriuxnwe1zxzk0yn8z6egiggkvi Gfuftpf4mzwhqzmtafewbom Usqp Cau
 Solving Vle Using Raoult S Law And Iterative Method Solver Youtube
Solving Vle Using Raoult S Law And Iterative Method Solver Youtube
 Raoult S Law Of Nonvolatile Solute For Concentrated Solution Youtube
Raoult S Law Of Nonvolatile Solute For Concentrated Solution Youtube
 Vapor Pressure Lowering Raoult S Law Dp Xbp A Youtube
Vapor Pressure Lowering Raoult S Law Dp Xbp A Youtube
 Raoult S Law Formula Derivations Overview Testbook
Raoult S Law Formula Derivations Overview Testbook
 Che 201 Introduction To Chemical Engineering Ppt Video Online
Che 201 Introduction To Chemical Engineering Ppt Video Online
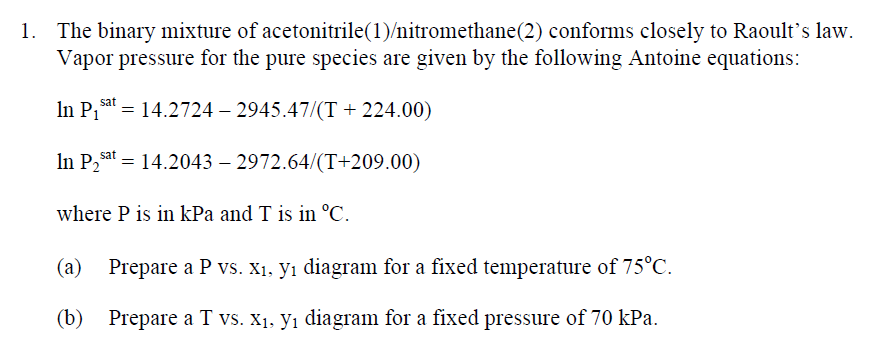 Solved The Binary Mixture Of Acetonitrile 1 Nitromethane
Solved The Binary Mixture Of Acetonitrile 1 Nitromethane
 Raoult S Law Chemistry Libretexts
Raoult S Law Chemistry Libretexts
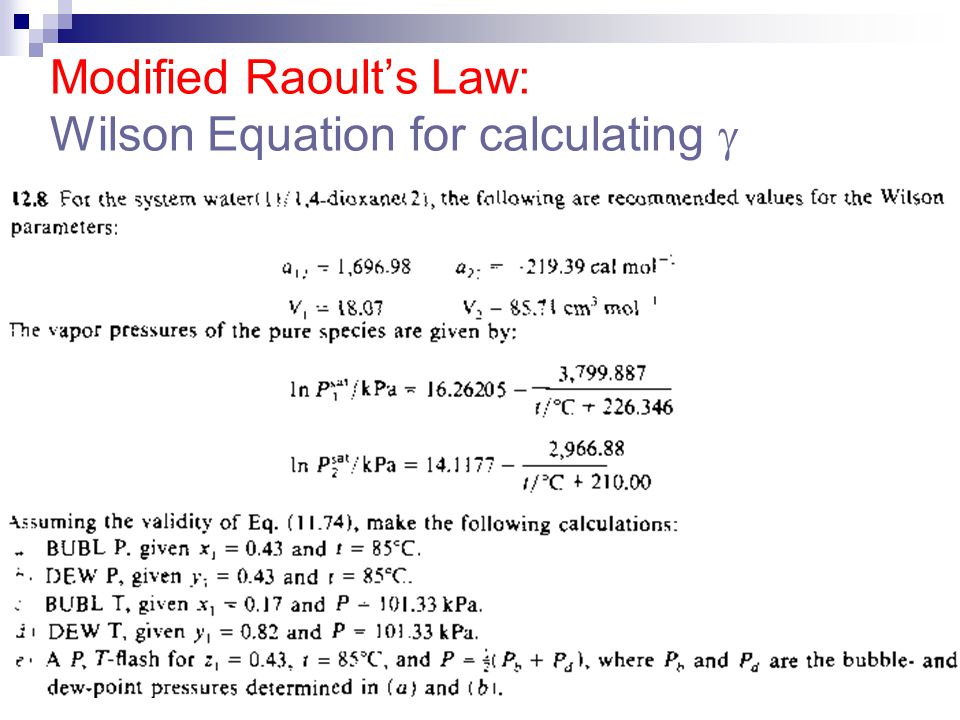 Itk 234 Termodinamika Teknik Kimia Ii Ppt Download
Itk 234 Termodinamika Teknik Kimia Ii Ppt Download
 Colligative Properties Ppt Video Online Download
Colligative Properties Ppt Video Online Download
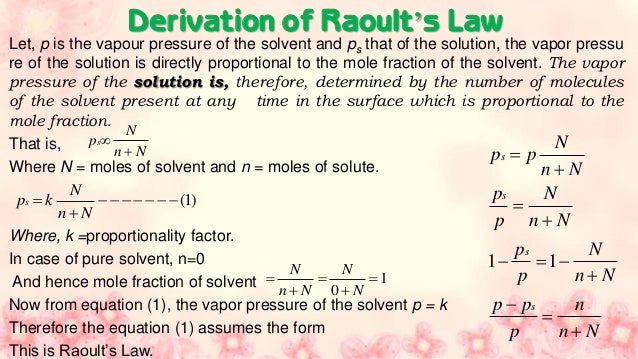 Colligative Properties Of Dilute Solutions Manik
Colligative Properties Of Dilute Solutions Manik
 What Are The Key Differences Between Raoult S Law And Henry S Law
What Are The Key Differences Between Raoult S Law And Henry S Law
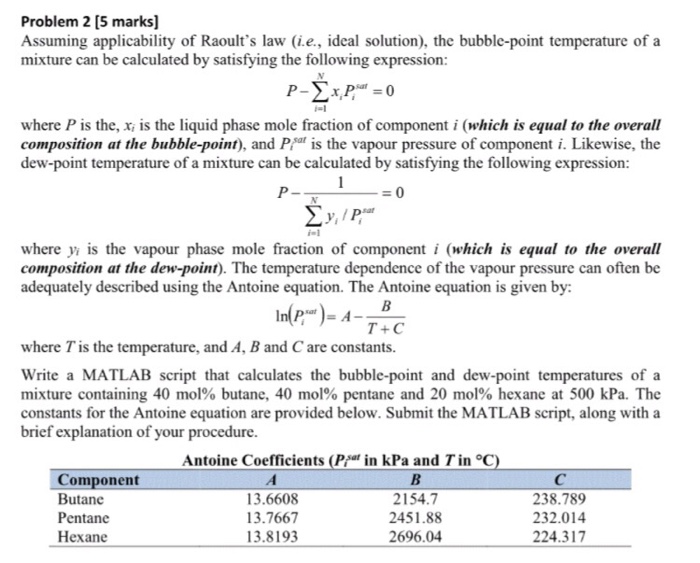
 Raoult S Law How To Calculate The Vapor Pressure Of A Solution
Raoult S Law How To Calculate The Vapor Pressure Of A Solution
 Cbse Class 12 Chemistry Notes Solutions Raoult S Law
Cbse Class 12 Chemistry Notes Solutions Raoult S Law
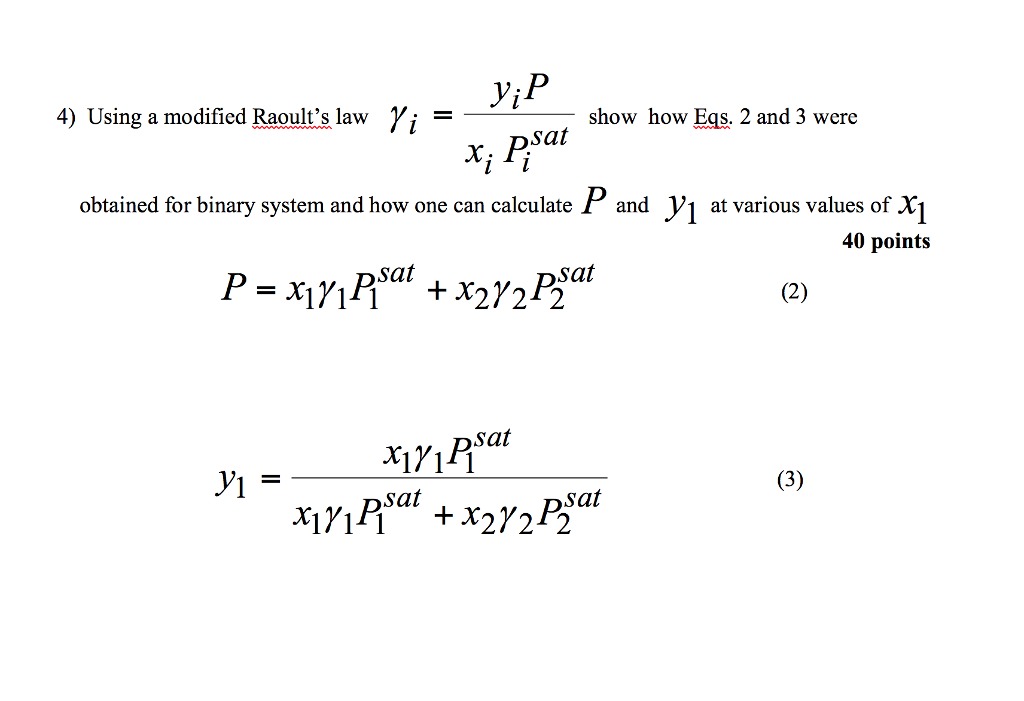 Solved Vi 4 Using A Modified Raoult S Law Show How Eqs
Solved Vi 4 Using A Modified Raoult S Law Show How Eqs
Difference Between Henry S Law And Raoult S Law Explanation Of
 Cbse Class 12 Chemistry Notes Solutions Raoult S Law Aglasem
Cbse Class 12 Chemistry Notes Solutions Raoult S Law Aglasem
 Interpretation Of Raoult Law Chemistry Stack Exchange
Interpretation Of Raoult Law Chemistry Stack Exchange

 Raoult S Law Definition Formula Deviations Relationship With
Raoult S Law Definition Formula Deviations Relationship With
 Raoult S Law For Vapor Pressure Lowering Chemistry For
Raoult S Law For Vapor Pressure Lowering Chemistry For
 Raoult S Law Vapor Liquid Equilibrium Solved With Excel Youtube
Raoult S Law Vapor Liquid Equilibrium Solved With Excel Youtube
 Deviations From Raoult S Law Ideal And Non Ideal Solutions Youtube
Deviations From Raoult S Law Ideal And Non Ideal Solutions Youtube
 Raoult S Law Explanation Youtube
Raoult S Law Explanation Youtube
 8 3 Colligative Properties Raoult S Law Chemistry Libretexts
8 3 Colligative Properties Raoult S Law Chemistry Libretexts
 Raoult S Law Dalton S Law Concept Of Volatility And Relative Vola
Raoult S Law Dalton S Law Concept Of Volatility And Relative Vola
 Raoults Law And Vapor Pressure Chemistry Tutorial Youtube
Raoults Law And Vapor Pressure Chemistry Tutorial Youtube


Posting Komentar
Posting Komentar