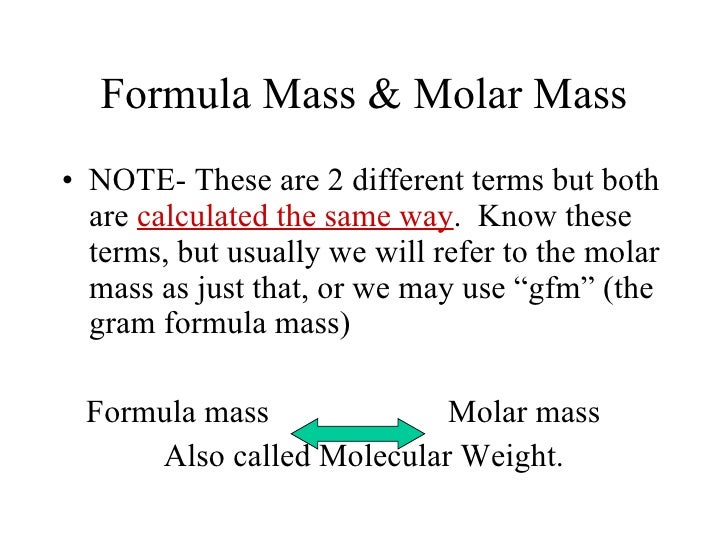
Formula Mass Vs Molar Mass
The above equation can convert u to g mol 1. But this quantity is in the unified mass unit u.
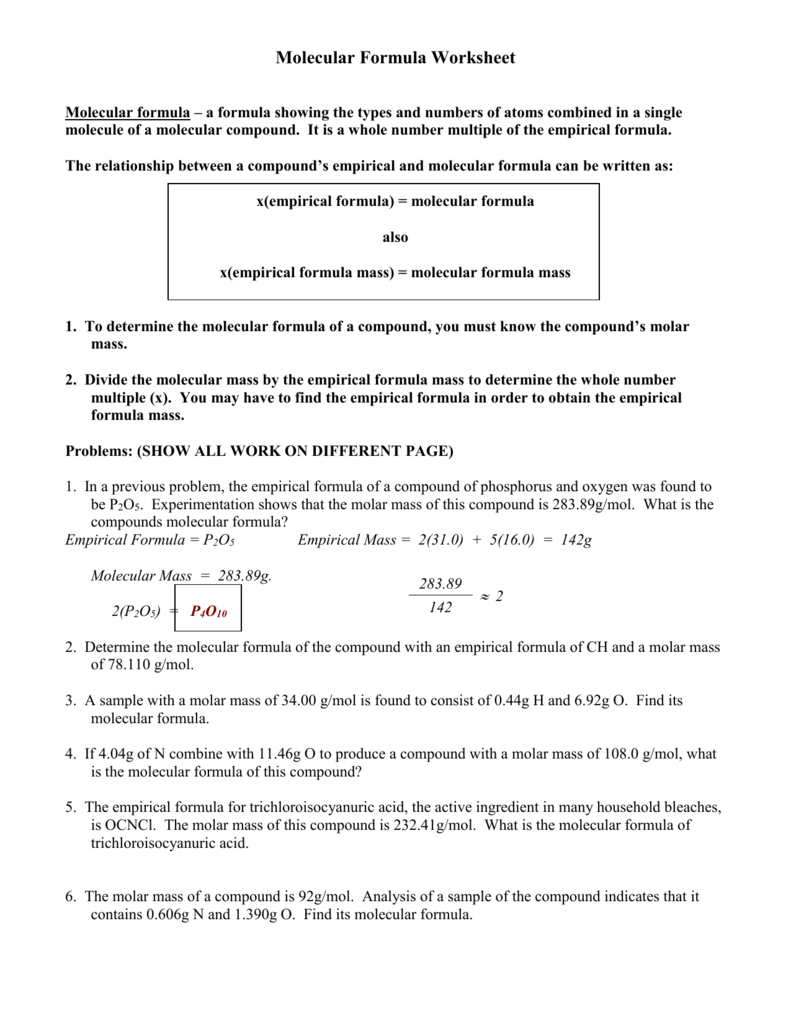
Empirical And Molecular Formulas
The molar mass of a substance is the mass in grams of 1 mole of the substance.
Formula mass vs molar mass. Formula mass is calculated for chemical compounds. The mass of one mole of carbon 12 atoms is exactly 12 grams which is its molar mass it is exactly 12 grams per mole. If you know the molecular weight you know the molar mass.
Molecular weight is the mass of one molecule of a substance while the molar mass is the mass of one mole of a substance. We can then use the calculated molar mass to convert between mass and number of moles of the substance. Terms like molecular mass and molecular weight are often used interchangeably with formula mass.
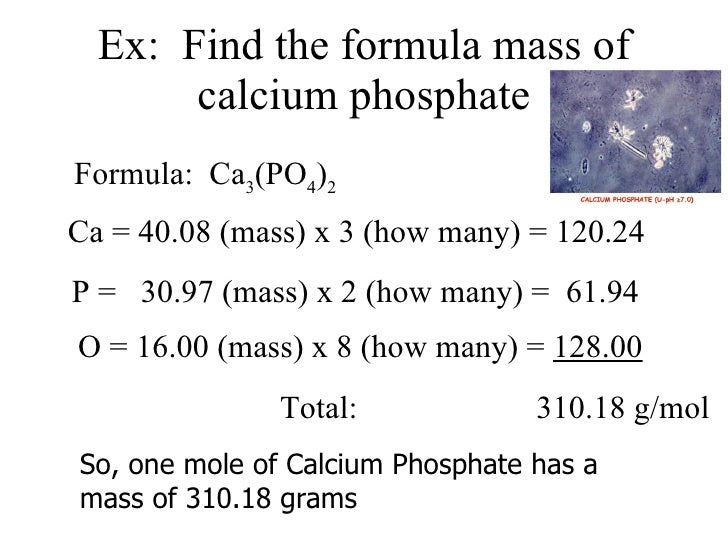
The units for molecular mass are given in atomic mass units while the units for molar mass are give in grams per mol. It is calculated using the empirical formula. The main difference between formula mass and the molecular mass is that formula mass is calculated by adding the masses of atoms present in the simplest formula that can be given for a molecule whereas molecular mass is calculated using the actual numbers of atoms present in a molecule.
Therefore the average atomic mass divided by one unified mass unit times the molar mass constant results in the molar mass. What is formula mass. The formula mass formula weight of a molecule is the sum of the atomic weights of the atoms in its empirical formula.
We can calculate the molar mass of molecules containing the same atom like o 2 or n 2 by multiplying the number of atoms by the molar mass of the atoms. The key difference between formula mass and molar mass is that the formula mass of a molecule or a compound is the sum of the atomic weights of the atoms in its empirical formula while molar mass is the mass in grams of 1 mol of substance what is formula mass. Whenever you see a molecular formula where you can divide the subscripts by a whole number usually 2 or 3 you know to expect the formula mass will be different.
As shown in this video we can obtain a substance s molar mass by summing the molar masses of its component atoms. Molar mass is the mass in grams of 1 mol of substance the number of particles in a mole is equal to 6 022 10 23. Molar mass is calculated for chemical substances that contain many elementary entities such as chemical.
As molecular mass is for molecules and compounds formula mass is for ionic compounds specially. The formula mass formula weight of glucose is 30 either no units or else grams per mole while the molecular mass molecular weight is 180 156 g mol. How to calculate the formula mass and molar mass of a compound.
So the mass of a mole of the atom is ma na. The molecular term just means you. So formula mass can be defined as the total mass of the ionic compound or the sum of individual mass of ions present.
How To Calculate Relative Formula Mass Relative Molecular Mass
Difference Between Atomic Mass And Molecular Mass لم يسبق له مثيل
 Solved The Molar Mass Of A Given Gas Can Be Obtained Thro
Solved The Molar Mass Of A Given Gas Can Be Obtained Thro
Atomic Mass Vs Molar Mass Arbuiso Com
 Empirical And Molecular Formulas Of Compounds Mole And Empirical
Empirical And Molecular Formulas Of Compounds Mole And Empirical
 How To Calculate Molar Mass 7 Steps With Pictures Wikihow
How To Calculate Molar Mass 7 Steps With Pictures Wikihow
 Ppt Chapter 7 The Mole Powerpoint Presentation Free Download
Ppt Chapter 7 The Mole Powerpoint Presentation Free Download
 01 Molecular Mass And Formula Mass Learn The Formula Unit
01 Molecular Mass And Formula Mass Learn The Formula Unit
 Compare Relative Molar Mass And Relative Formula Unit Mass
Compare Relative Molar Mass And Relative Formula Unit Mass
 Calculating Molar Mass And Number Of Moles Worked Example Video
Calculating Molar Mass And Number Of Moles Worked Example Video
:max_bytes(150000):strip_icc()/200257396-001-56a12e8f5f9b58b7d0bcd74b.jpg) Formula Mass Definition And Example Calculation
Formula Mass Definition And Example Calculation
How To Calculate Molar Mass Howwiki Pro
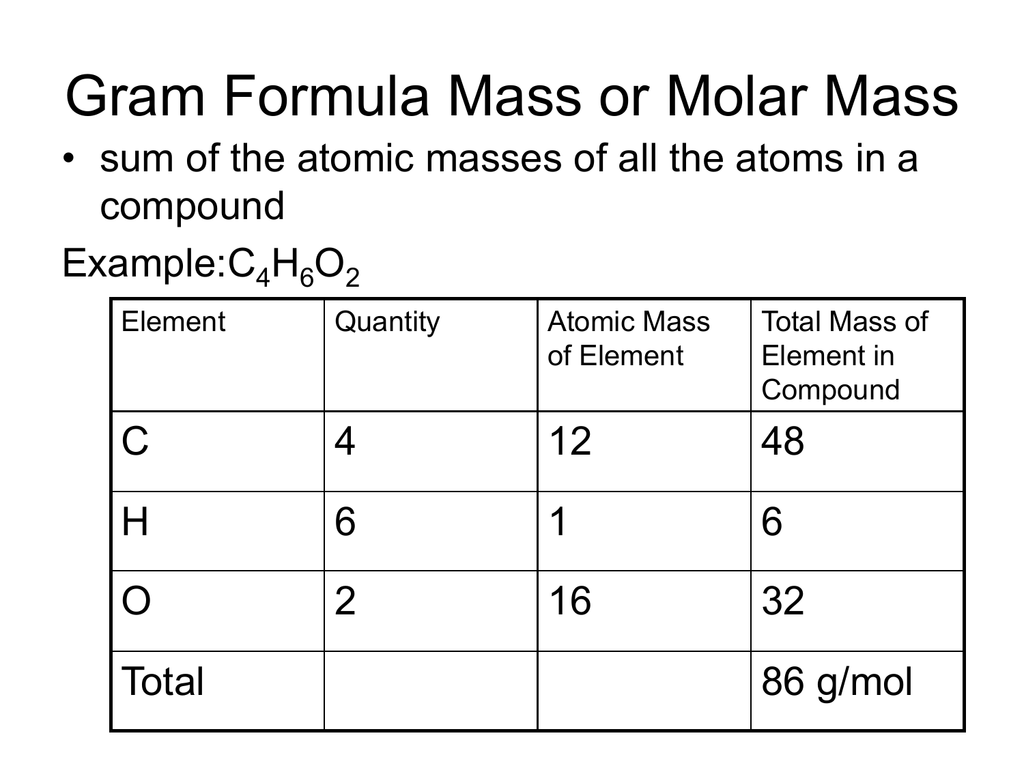 Gram Formula Mass Or Molar Mass
Gram Formula Mass Or Molar Mass
 Ppt Atomic Weight Molecular Weight Formula Weight And Molar
Ppt Atomic Weight Molecular Weight Formula Weight And Molar
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcrioatj0risjg9ydcdnrcuvwxnexlogxx2ygwiduqai0frx4yiv Usqp Cau
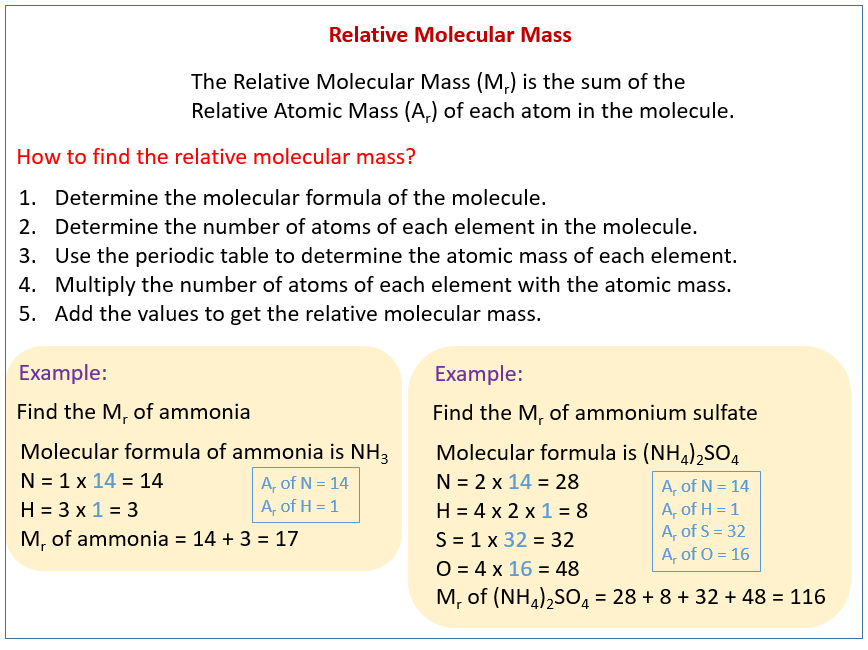 Relative Molecular Mass Relative Formula Mass Solutions
Relative Molecular Mass Relative Formula Mass Solutions
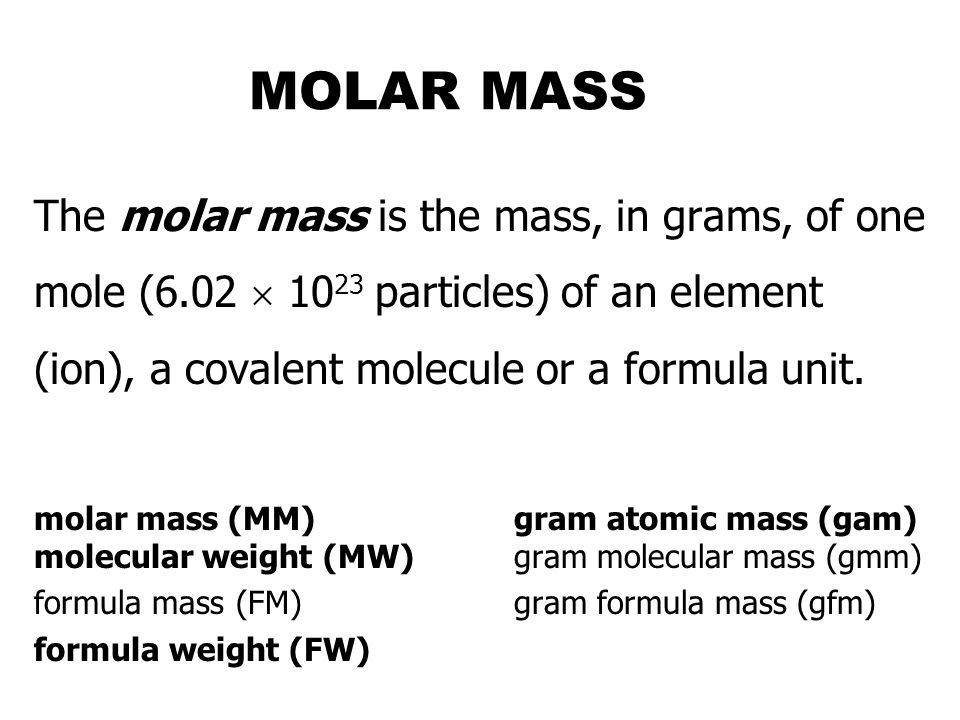 Molar Mass The Molar Mass Is The Mass In Grams Of One Mole 6 02
Molar Mass The Molar Mass Is The Mass In Grams Of One Mole 6 02
Difference Between Molar Mass And Molecular Weight Definition
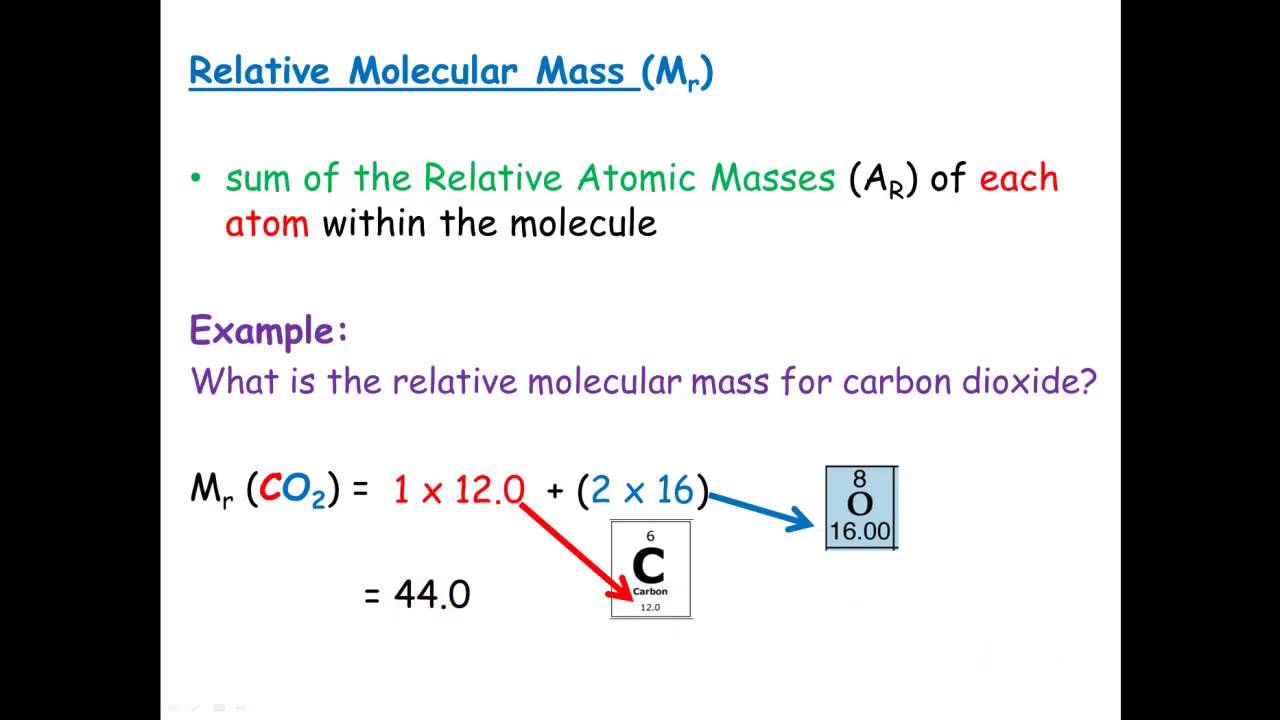 Relative Molecular Mass Relative Formula Mass Youtube
Relative Molecular Mass Relative Formula Mass Youtube
 Molecular Weight Calculator Boc Sciences
Molecular Weight Calculator Boc Sciences
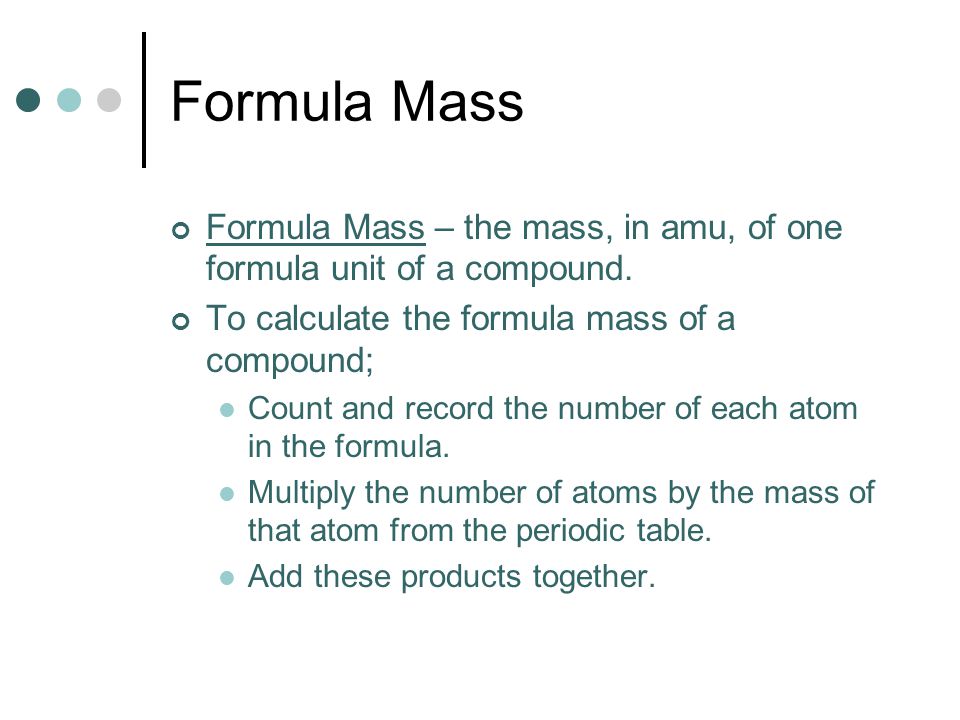 Molar Mass Percent Composition Empirical And Molecular Formulas
Molar Mass Percent Composition Empirical And Molecular Formulas
Difference Between Formula Mass And Molecular Mass Definition
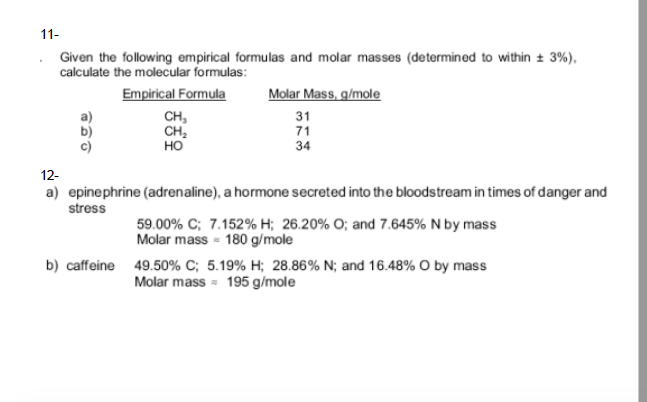 Solved Given The Following Empirical Formulas And Molar M
Solved Given The Following Empirical Formulas And Molar M
Difference Between Molar Mass And Molecular Mass Difference Between
 Chapter03 Knight Part2 Formula Mass The Mass Of An Individual
Chapter03 Knight Part2 Formula Mass The Mass Of An Individual
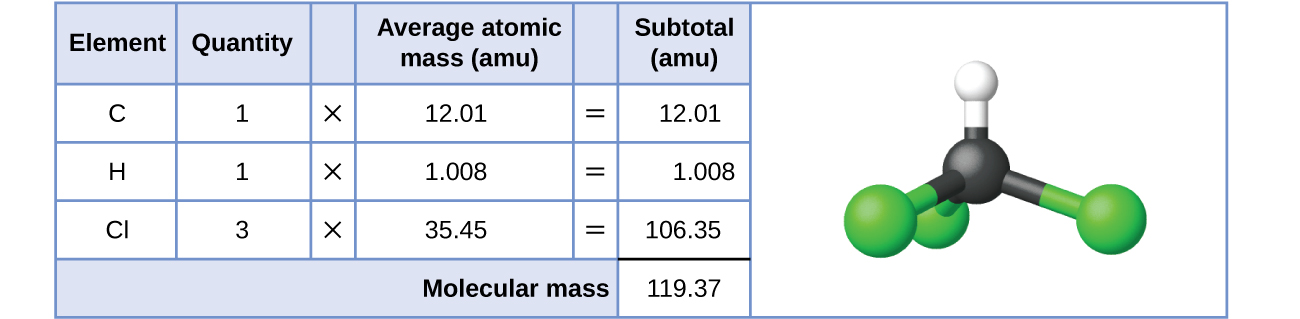 3 1 Formula Mass And The Mole Concept Chemistry
3 1 Formula Mass And The Mole Concept Chemistry
 Relative Molecular Mass Relative Formula Mass Solutions
Relative Molecular Mass Relative Formula Mass Solutions
 The Mole Unit 6 Formula Mass Formula Mass Also Called Gram
The Mole Unit 6 Formula Mass Formula Mass Also Called Gram
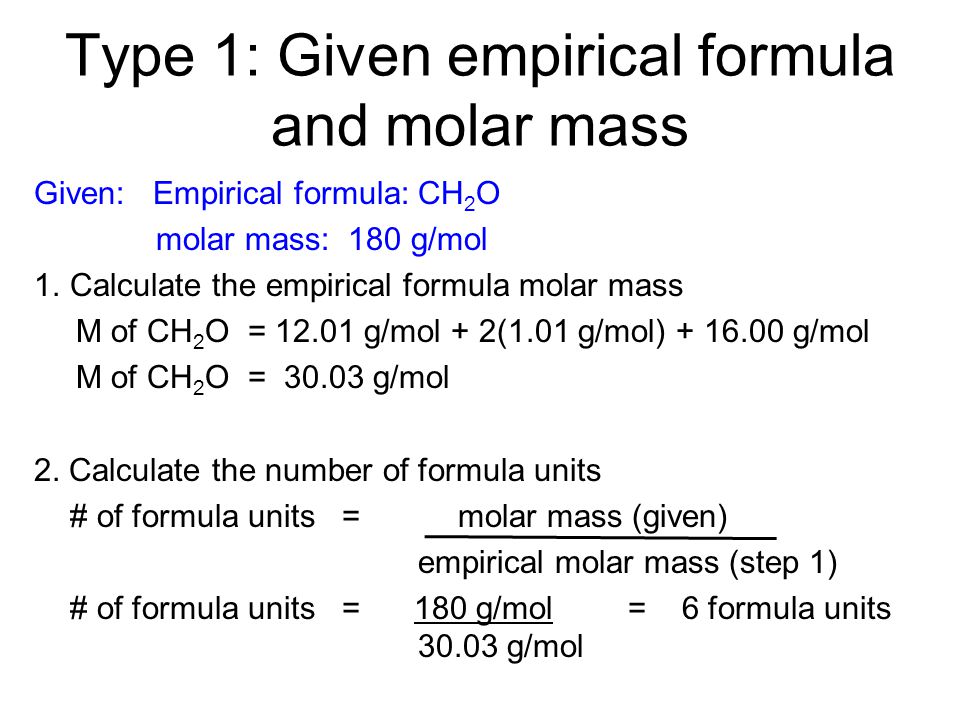 How To Determine Molecular Formula Molar Mass لم يسبق له مثيل
How To Determine Molecular Formula Molar Mass لم يسبق له مثيل
 Mcat Atomic Mass Unit Molecular Mass Formula Mass Mole Molar
Mcat Atomic Mass Unit Molecular Mass Formula Mass Mole Molar
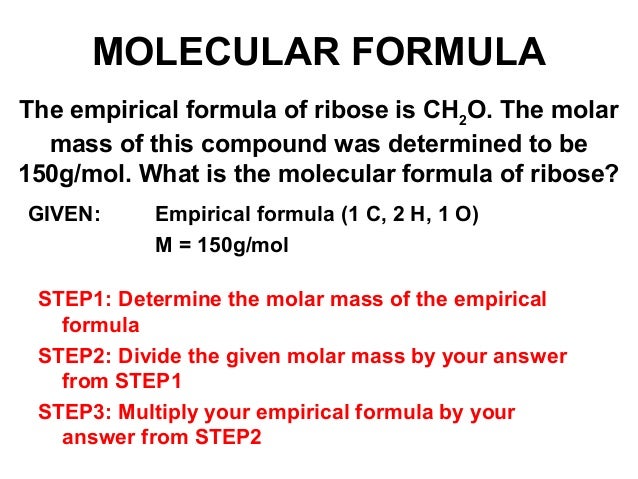 How To Calculate Molar Mass Howwiki Pro
How To Calculate Molar Mass Howwiki Pro
:max_bytes(150000):strip_icc()/GettyImages-545862141-58d6b12d3df78c5162f4104e.jpg) Formula Mass Weight Versus Molecular Mass
Formula Mass Weight Versus Molecular Mass
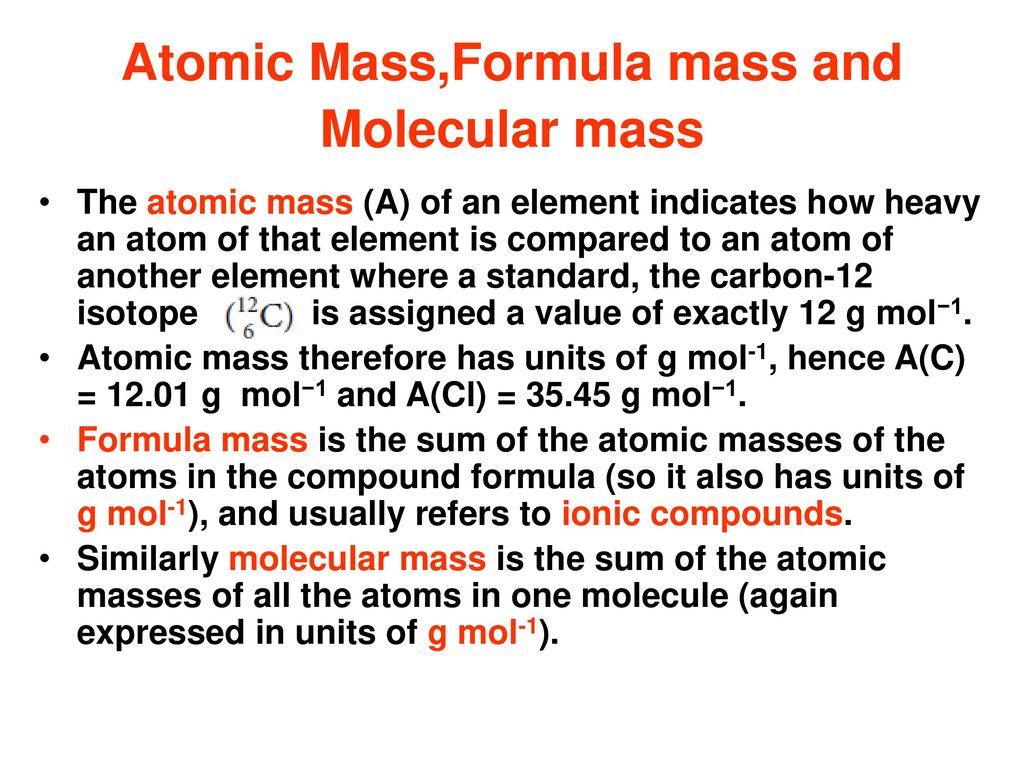 Atomic Mass Formula Mass And Molecular Mass Ppt Download
Atomic Mass Formula Mass And Molecular Mass Ppt Download
 Gram Formula Mass Worksheet Worksheets Cells Worksheet
Gram Formula Mass Worksheet Worksheets Cells Worksheet
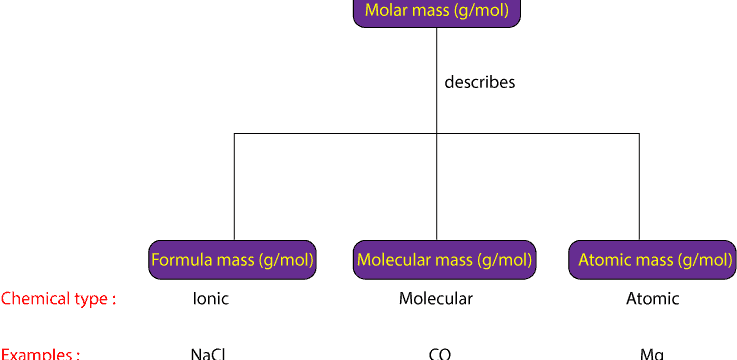 What Is Molar Mass And How Do You Calculate It
What Is Molar Mass And How Do You Calculate It
Posting Komentar
Posting Komentar